FDA Approved Opdualag: The First Immunotherapy Targeting LAG-3
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
FDA Approved Opdualag: The First Immunotherapy Targeting LAG-3
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
FDA Approved Opdualag: The First Immunotherapy Targeting LAG-3
The FDA has approved the combination of Relatlimab and Nivolumab for the treatment of advanced melanoma patients, which will be marketed under the new name Opdualag. Both drugs are immune checkpoint inhibitors, with Relatlimab targeting the LAG-3 protein and Nivolumab targeting the PD-1 protein, aiming to unleash the immune response by blocking these proteins.
Relatlimab is the first drug approved by the FDA to block LAG-3 activity. Unlike other FDA-approved immune checkpoint inhibitors, patients will receive a combination of the two drugs through intravenous infusion of Opdualag. FDA has approved the combination of Nivolumab and Relatlimab for patients aged 12 and above with previously untreated melanoma that cannot be surgically removed or has spread (metastasized) in the body.
The RELATIVITY-047 clinical trial studied the combination therapy of Nivolumab and Relatlimab in melanoma patients, showing that patients receiving the combination had a longer progression-free survival than those receiving Nivolumab alone (10.1 months vs. 4.6 months). Common side effects include fatigue, rash, joint pain, and diarrhea, with 14.6% of patients stopping the trial drug due to side effects. However, more follow-up is needed to confirm if this combination can prolong patients’ overall survival.
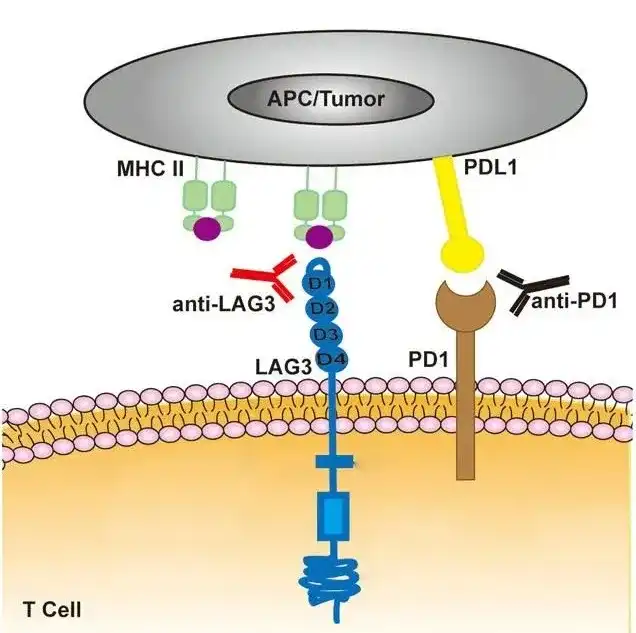
Figure 1: Opdualag treatment involves receiving two antibody drugs in the same infusion. One drug blocks the immune checkpoint protein LAG-3, and the other blocks the checkpoint protein PD-1.
Expanding to Other Cancer Indications
Opdualag is being evaluated for the treatment of multiple myeloma, esophageal or gastric cancer, sarcoma, and other types of cancer. Additionally, Opdualag is also being studied in clinical trials for other cancers, including lung, colorectal, and liver cancer. The NCI-MATCH trial has added a new treatment arm to evaluate the effectiveness of Opdualag in patients with cancer progression after receiving immune checkpoint inhibitor therapy.
Studies suggest that for certain cancers, combination immune checkpoint inhibitors, such as Nivolumab and Ipilimumab, are particularly effective in treating melanoma that has spread to the brain. This combination can block checkpoint proteins on immune cells, enhancing treatment efficacy.
Previously, the combination of Nivolumab and Ipilimumab was the standard treatment for inoperable or metastatic melanoma. In terms of progression-free survival, the combination of Nivolumab plus Ipilimumab and the combination of Relatlimab plus Nivolumab showed similar results in clinical trials, with the Relatlimab-Nivolumab regimen appearing to have fewer side effects. Less than 20% of patients receiving Relatlimab-Nivolumab reported serious side effects, compared to nearly 60% of patients receiving Nivolumab-Ipilimumab. Therefore, the Relatlimab-Nivolumab regimen is more effective.
In conclusion:
In the treatment selection for advanced melanoma, it is crucial to consider the safety and overall survival rates of treatment regimens. This study aims to observe whether the combination of Nivolumab and Relatlimab can improve patient survival rates and hopes to obtain longer follow-up data from this trial to support patient and physician decision-making.
FDA Approved Opdualag: The First Immunotherapy Targeting LAG-3
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



