Breakthroughs in Immunotherapy for Melanoma: Checkpoint Blockade and Beyond
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Breakthroughs in Immunotherapy for Melanoma: Checkpoint Blockade and Beyond
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Breakthroughs in Immunotherapy for Melanoma: Checkpoint Blockade and Beyond
Melanoma, the most aggressive and deadly skin cancer, has seen significant advancements in immunotherapy, particularly through checkpoint blockade treatments. Studies indicate that melanoma is an immunoreactive tumor often associated with increased ultraviolet exposure and elevated tumor mutational burden (TMB), contributing to enhanced immunogenicity.
The extent of reactive lymphocyte infiltration around tumors correlates with better prognosis, and the classification of melanoma based on tumor-infiltrating lymphocyte (TIL) distribution (active, inactive, or absent) is still in use.
Since 2011, the immunotherapy checkpoint inhibitor ipilimumab has been approved for advanced melanoma, marking thirteen years of being the first treatment to extend melanoma survival.
Subsequently, in 2014, another critical T-cell immune checkpoint PD-1 inhibitors, pembrolizumab and nivolumab, were approved for treating metastatic melanoma.
Despite the clinical success of immune checkpoint blockade (ICB) in melanoma, a significant portion of patients still cannot achieve long-term benefits, even with optimal combination therapies.
This underscores the need for better predictive biomarkers and new rational targets to overcome immune resistance.
Now is the time to review the lessons learned in modulating the immune system to treat cancer and explore new approaches to enhance the effectiveness of current and emerging immunotherapies.
Melanoma-Specific T-Cell Responses
Melanoma is rich in tumor-infiltrating lymphocytes (TILs) specific to melanoma-associated antigens, indicating that anti-melanoma T cells can undergo activation, expansion, and then re-recruitment into the tumor. The intrinsic T-cell response to melanoma has been utilized for various therapies, including identifying homologous antigens for vaccine development and expanding and/or engineering tumor-specific T cells for adoptive cell therapy (ACT).
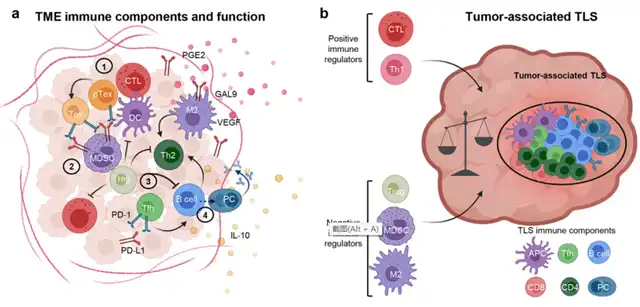
Since the discovery of IL-2 in 1977, allowing T cells to expand ex vivo for characterization, research on melanoma TILs has advanced. T cells recognizing melanoma-associated tumor antigens, such as cancer embryonic antigens (e.g., Mage-A1, NY-ESO-1), melanocyte differentiation antigens (e.g., MART-1, gp100, tyrosinase), overexpressed antigens (e.g., PRAME), new antigens originating from tumor-specific somatic cell mutations, and other sources, have been identified. The heterogeneity in B cells and their antibody products in melanoma patients may explain contradictory independent research results regarding B cell infiltration and negative prognosis.
TLS in melanoma is highly dynamic and can attract immunosuppressive cells, such as regulatory T cells (Tregs), immature tolerogenic dendritic cells (DC), and/or myeloid-derived suppressor cells (MDSC), to counteract excessive inflammation. Precisely measuring the immunostimulatory and immunosuppressive potential of tumor-associated TLS and predicting their fate based on components and inflammatory signals could provide valuable biomarkers for immunotherapy responses. Recent reports suggest that TLS and the biology of B cells infiltrating tumors are associated with improved responses to ICB.
Immunosuppressive Mechanisms in Melanoma
Despite the immunogenicity of melanoma, metastatic melanoma typically does not spontaneously regress. Strong immune selection pressure on melanoma’s immunogenicity can induce tumor adaptation and suppression of anti-tumor immunity. Additionally, local inflammation can activate steady immune feedback, aiding in adaptive resistance. Melanoma cells can directly attract immunosuppressive cells, especially through MHC-II expression, interacting with immunosuppressive CD4+ T cell subsets. Melanoma patients show increased Tregs in peripheral blood, lymph nodes, and the tumor microenvironment (TME), suppressing TIL function.
The latest advancements in ICB therapy for melanoma
The first immune checkpoint inhibitor applied to melanoma treatment was ipilimumab, targeting CTLA-4, approved in 2011 for metastatic melanoma. Subsequently, in 2014, PD-1 inhibitors pembrolizumab and nivolumab were approved for metastatic melanoma. Considering the differences and potential complementary effects of CTLA-4 and PD-1 inhibitors, combination trials demonstrated their long-term efficacy in metastatic melanoma, with 49% of patients still surviving after 6.5 years, albeit with increased toxicity. Current research focuses on alternative and/or dose-reduced regimens for anti-PD-1 + anti-CTLA-4 to lower toxicity.
The discovery of immune inhibitory receptors in exhausted T cells has spurred the development of antibodies against new immune checkpoint molecules. The most promising new ICB target is LAG-3, similar in structure to CD4, a surface inhibitory receptor that competitively binds to MHC-II and other ligands (e.g., galectin-3). While LAG-3 inhibitors as monotherapy show moderate anti-tumor efficacy, combined anti-LAG-3 + anti-PD-1 exhibits significantly enhanced therapeutic activity in several mouse tumor models, including melanoma.
Relatlimab, the most advanced anti-LAG-3 antibody, was FDA approved in March of this year in a fixed-dose combination with nivolumab for treating unresectable or metastatic melanoma in adults and children aged 12 and older. Clinical trial results indicate higher progression-free survival (PFS) with relatlimab + nivolumab, with PFS of 10.1 months compared to 4.6 months with nivolumab monotherapy.
Other advancements in ICB therapy for melanoma come from studies on its application in early-stage disease, either post-surgical resection (adjuvant therapy) or pre-surgical resection (neoadjuvant therapy). Ipilimumab was the first ICB therapy to demonstrate persistent survival benefits in adjuvant melanoma therapy, followed by PD-1 blockade with nivolumab or pembrolizumab, showing improved recurrence-free survival (RFS) in high-risk stage III patients compared to placebo or even ipilimumab. PD-1 inhibitors have become the standard care for adjuvant therapy due to improved toxicity compared to ipilimumab. Recently, pembrolizumab’s adjuvant therapy gained FDA approval for stage II/C melanoma.
Neoadjuvant ICB therapy has also made progress, with five completed studies in melanoma to date. Neoadjuvant ipilimumab + nivolumab or single-agent PD-1 blockade showed pathological complete response (pCR) rates of 33-57% and 19-25%, respectively. Alternative combination regimens, such as neoadjuvant nivolumab + relatlimab, demonstrated an impressive pCR rate of 59%.
Targeting Melanoma Metabolism to Overcome Immunotherapy Resistance
Despite the success of ICB, the efficacy of these therapies, even in combination, has reached a limit, necessitating new drugs. Tumor cells often adapt to aerobic glycolysis, giving them a metabolic advantage over normal immune cells in the TME, favoring tumor progression and immune evasion. Targeting tumor metabolism is becoming a crucial focus for combination immunotherapy.
Metabolic competition is particularly relevant in melanoma, where increased glycolysis in human melanoma correlates negatively with T-cell infiltration, activation, and responses to ACT or ICB. Anti-diabetic drugs like metformin are being
studied in melanoma to counteract this high glycolytic phenotype, with preliminary retrospective analysis showing a reduced incidence of new brain metastases in patients receiving metformin during ICB treatment, indicating a favorable prognosis.
Moreover, reduced oxygen tension in the high oxidative tumor microenvironment can promote the reactivation of T-cell exhaustion and resistance to T-cell PD-1 inhibition. Conversely, tumor glycolysis and glucose deprivation have a preferential effect on CTLA-4 blockade. Oxidative and glycolytic tumor metabolism respectively opposes resistance to PD-1 and anti-CTLA-4 inhibitors, explaining, at least in part, the different cellular localization of these immune therapies’ direct targets. PD-1 blockade primarily acts on reinvigorating dysfunctional PD-1+ T cells, while CTLA-4 blockade acts against Tregs, stabilizing their suppressive function in glucose-deprived environments. Recent studies suggest that targeting lactate or fatty acid metabolism in Tregs can enhance mouse melanoma models’ response to ICB.
In Conclusion
Immune checkpoint inhibitors have been approved for advanced melanoma treatment for a decade.
Over these years, as our understanding of the human melanoma immune microenvironment deepens, we have made significant progress in exploring new drugs and therapies based on immune checkpoint inhibitors.
However, with an increasing number of drugs available, we need to elucidate the mechanisms of combination immunotherapy to guide rational combinations.
Attention should be given to robust biomarkers, resistance mechanisms, and the combination with other therapies.
Looking to the future, neoadjuvant therapy shows promise.
Additionally, the toxicity of current and new immune therapy combinations remains a critical point to address, and understanding the molecular mediators of immune toxicity will greatly help control these side effects and improve patient management.
Breakthroughs in Immunotherapy for Melanoma: Checkpoint Blockade and Beyond
References:
1.A decade of checkpoint blockade immunotherapy in melanoma: understanding the molecular basis for immune sensitivity and resistance. Nat Immunol. 2022 May; 23(5): 660–670.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



