Keytruda Treatment for Advanced Melanoma: 7-Year Follow-Up Shows Over 1/3 of Patients Achieve 7-Year Survival
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Keytruda Treatment for Advanced Melanoma: 7-Year Follow-Up Shows Over 1/3 of Patients Achieve 7-Year Survival
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Keytruda Treatment for Advanced Melanoma: 7-Year Follow-Up Shows Over 1/3 of Patients Achieve 7-Year Survival.
Immune checkpoint inhibitors (ICIs) have seen rapid development in recent years, with their reach extending to the treatment of virtually all solid tumors.
However, when we talk about where the dream of ICIs began, many still remember melanoma.
On March 25, 2011, the U.S. Food and Drug Administration (FDA) approved pembrolizumab injection as monotherapy for the treatment of unresectable or metastatic melanoma patients who couldn’t undergo surgery. Pembrolizumab became the first ICI to be marketed globally.
Subsequently, other ICIs like nivolumab and ipilimumab, collectively known as the “PD-1 Duo,” also received FDA approval for melanoma as their first indication.
Following the release of 7.5-year follow-up data from the CheckMate 067 study for nivolumab in advanced melanoma in 2022, Keytruda (pembrolizumab) has now published 7-year follow-up data from the KEYNOTE-006 study for advanced melanoma in the official ASCO journal JCO.
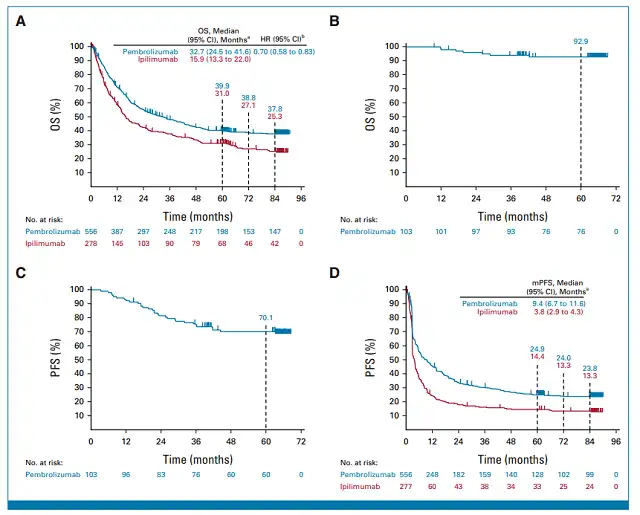
Figure 1: 7-year follow-up PFS and OS results
The KEYNOTE-006 study was an open-label, randomized Phase III trial designed to compare the efficacy and safety of pembrolizumab and ipilimumab in patients with advanced melanoma. The primary eligibility criteria for patients included unresectable stage III or stage IV melanoma, ≤1 prior therapy excluding CTLA-4, PD-1, or PD-L1 drugs, known BRAF status, and a performance status score of 0 or 1. All patients were randomized 1:1:1 to receive pembrolizumab 10 mg/kg IV every 2 weeks, pembrolizumab 10 mg/kg IV every 3 weeks, or ipilimumab 3 mg/kg IV every 3 weeks for a duration of 2 years.
Participants in the KEYNOTE-006 study who received second-line treatment with pembrolizumab, including those who initially achieved disease stability or better in the first-line treatment but later experienced disease progression, entered the KEYNOTE-587 study for second-line treatment.
The primary endpoint of the KEYNOTE-587 study was overall survival (OS). In total, 834 patients were enrolled in the treatment (556 in the pembrolizumab group and 278 in the ipilimumab group), with 210 patients subsequently included in the KEYNOTE-587 trial (158 in the pembrolizumab group and 52 in the ipilimumab group).
The 7-year follow-up results of the KEYNOTE-006 study showed that pembrolizumab significantly extended overall survival. The median OS for the pembrolizumab group was 32.7 months compared to 15.9 months for the ipilimumab group (HR, 0.70; 95% CI: 0.58-0.83). The 7-year OS rates were 37.8% for pembrolizumab vs. 25.3% for ipilimumab. Patients who received pembrolizumab as first-line treatment also had an extended OS (HR, 0.67; 95% CI, 0.53-0.84), with a 7-year OS rate of 41.2% compared to 27.6% for ipilimumab. Pembrolizumab significantly improved progression-free survival (PFS), with median PFS of 9.4 months for pembrolizumab vs. 3.8 months for ipilimumab (HR, 0.62; 95% CI, 0.50-0.76). The 7-year PFS rates were 23.8% for pembrolizumab and 13.3% for ipilimumab. Patients who completed ≥94 weeks of pembrolizumab treatment and achieved disease stability (SD) or better had higher survival rates, with 5-year OS rates and PFS rates of 92.9% and 70.1%, respectively.
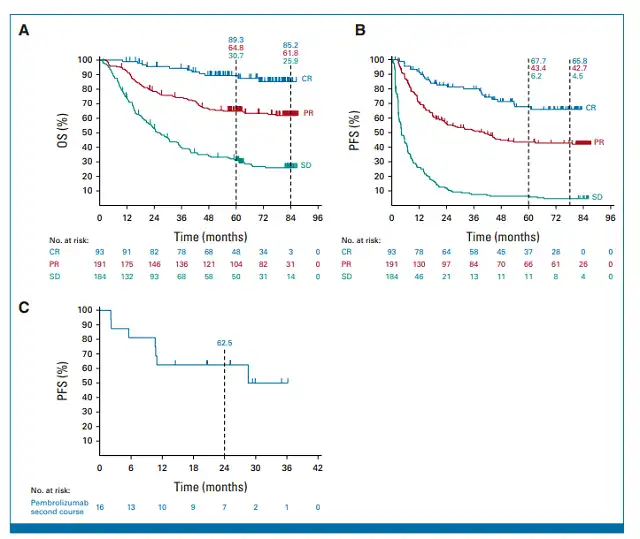
Figure 2 : Subgroup analysis results of K drug group
In the study, 93 patients (16.7%) in the pembrolizumab group achieved a complete response (CR), 191 patients (34.4%) had a partial response (PR), and 184 patients (33.1%) maintained stability (SD). When assessing outcomes based on CR/PR/SD response categories, both PFS and OS were unsurprisingly best in the CR group, followed by PR and SD. The 7-year OS rates for these three groups were 85.5% vs. 61.8% vs. 25.9%, and the 7-year PFS rates were 65.8% vs. 42.7% vs. 4.5%. Sixteen patients who achieved CR or PR in the first round of treatment with pembrolizumab received a second course of treatment, with a 2-year PFS rate of 62.5%. Patients who responded to the first round of pembrolizumab treatment with CR or PR and subsequently received a second course of pembrolizumab treatment had better outcomes.
In summary, the 7.5-year follow-up data from the CheckMate 067 study for nivolumab in advanced melanoma showed a median OS of 36.9 months and a 7.5-year OS rate of 42%. The 7-year follow-up data from the KEYNOTE-006 study for pembrolizumab showed median OS of 32.7 months and a 7-year OS rate of 37.8%, with a 7-year OS rate of 41.2% for first-line pembrolizumab treatment. Both clinical trials included over one-third of PD-1-treated patients who achieved 7-year survival, supporting the long-term survival benefit of PD-1 in advanced melanoma and its use as first-line treatment.
Expert Comments:
In August 2023, the Journal of Clinical Oncology (JCO) published the 7-year follow-up results of Keynote 006, providing further data support for the PD-1 antibody pembrolizumab in inducing long-term survival in patients with advanced melanoma.
The Keynote 006 study enrolled 834 patients with advanced melanoma who had either progressed after first-line treatment or failed non-immunotherapy drugs. These patients were randomized into three groups: pembrolizumab (10 mg/kg every 2 weeks), pembrolizumab (10 mg/kg every 3 weeks), and ipilimumab (3 mg/kg every 3 weeks). Patients achieving disease control (DCR) after 2 years of pembrolizumab or 4 cycles of ipilimumab were observed without treatment, while those with tumor progression could receive pembrolizumab again for up to 1 year. Since there was no difference in efficacy between the two pembrolizumab dose groups, they were combined for analysis. After a minimum of 7 years of follow-up, the 7-year survival rate in the pembrolizumab group was 37.8%, while in the ipilimumab group, it was 25.3%.
The key characteristics associated with long-term survival in the pembrolizumab group are as follows:
1. Completion of 2 years of pembrolizumab treatment: Patients who completed 2 years (94 weeks) of pembrolizumab treatment had a remarkable 5-year survival rate of 92.9% and a 5-year progression-free survival (PFS) of 70.1%. In this group, patients with BRAF mutations, those who had previously received BRAF and MEK inhibitor combination therapy, elevated LDH levels, large lesions, and brain metastases all benefited from pembrolizumab treatment.
2. Tumor shrinkage during pembrolizumab treatment as an early predictor of long-term survival: The 7-year survival rates for patients achieving the best response to pembrolizumab treatment (complete response, partial response, and stable disease) were 85.2%, 61.8%, and 25.9%, respectively. Patients who achieved complete or partial response with the initial pembrolizumab treatment had a favorable response to subsequent treatment with pembrolizumab. Tumor shrinkage also predicted long-term survival in patients treated with nivolumab or nivolumab in combination with ipilimumab in the Checkmate 067 study.
3. The 5-year survival rate as a predictor of long-term survival: The 5-year, 6-year, and 7-year survival rates in the pembrolizumab group were 39.9%, 38.8%, and 37.8%, respectively. Similar survival characteristics were observed in the nivolumab-treated group in the Checkmate 067 study, with a 5-year survival rate of 44%. These survival curves entered a plateau phase in the fourth year. In summary, Keynote 006 and Checkmate 067 studies demonstrate that tumor shrinkage during treatment with PD-1 antibodies or PD-1 antibodies in combination with CTLA-4 antibodies is a crucial factor for long-term survival in patients with advanced melanoma. According to RECIST criteria, a tumor shrinkage exceeding 30% is considered a partial response (PR). Other studies suggest that even a tumor shrinkage of more than 10% after PD-1 antibody treatment can lead to long-term survival.
Factors Influencing PD-1 Antibody Efficacy:
A retrospective analysis identified several key factors that influence the efficacy of PD-1 antibodies. These include the histological subtype of melanoma, the location of metastatic lesions, tumor size, LDH levels, and performance status (PS). Regarding the location of metastatic lesions and tumor size, the efficacy is significantly higher for lung and lymph node metastases compared to liver metastases, and asymptomatic brain metastases have a higher response rate than symptomatic brain metastases. Tumors with a diameter larger than 10 cm show a significantly reduced response rate. In terms of melanoma histological subtype, the efficacy is lower in Asian populations compared to Caucasians, and acral and mucosal melanomas have lower efficacy compared to cutaneous melanomas. Furthermore, a substantial amount of clinical research data suggests that within the dose range of 0.3 mg/kg to 10 mg/kg, the efficacy of PD-1 antibodies is not dose-dependent, a point supported by the Keynote 006 study.
UV exposure is not a primary trigger for melanoma in China (cutaneous, acral, and mucosal types). These melanomas have a low tumor mutation burden, which may be one of the reasons why single-agent PD-1 antibody therapy is less effective in Chinese melanoma patients.
References:
1. Seven-Year Follow-Up of the Phase III KEYNOTE-006 Study: Pembrolizumab Versus Ipilimumab in Advanced Melanoma. J Clin Oncol. 2023 Aug 20;41(24):3998-4003. doi: 10.1200/JCO.22.01599. Epub 2023 Jun 22.
2. Long-Term Outcomes With Nivolumab Plus Ipilimumab or Nivolumab Alone Versus Ipilimumab in Patients With Advanced Melanoma. J Clin Oncol. 2022 Jan 10;40(2):127-137.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



