NCCN guide updated: Nivolumab still expected to “cure” liver cancer
- A Single US$2.15-Million Injection to Block 90% of Cancer Cell Formation
- WIV: Prevention of New Disease X and Investigation of the Origin of COVID-19
- Why Botulinum Toxin Reigns as One of the Deadliest Poisons?
- FDA Approves Pfizer’s One-Time Gene Therapy for Hemophilia B: $3.5 Million per Dose
- Aspirin: Study Finds Greater Benefits for These Colorectal Cancer Patients
- Cancer Can Occur Without Genetic Mutations?
NCCN guide updated: Nivolumab still expected to “cure” liver cancer
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
NCCN guide updated: Nivolumab still expected to “cure” liver cancer.
Recently, the NCCN Guidelines for Hepatobiliary Tumors have been updated to version 2021 V4. This update is mainly limited to the use of nivolumab in the treatment of liver cancer.
Previously, after consultation with the U.S. Food and Drug Administration (FDA), Bristol-Myers Squibb voluntarily withdrew Opdivo (nivolumab) as an indication for the second-line treatment of hepatocellular carcinoma (HCC) patients. This decision was made after regulatory agencies evaluated checkpoint inhibitors that had passed accelerated approval but eventually failed in confirmatory clinical trials.
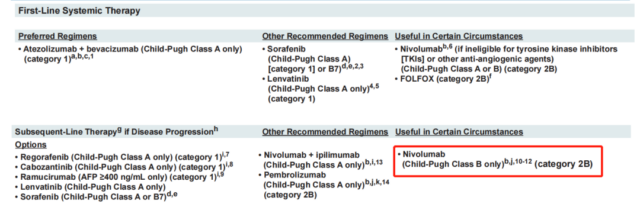
In response to the withdrawal of this indication, the guidelines also adjusted the status of nivolumab in the treatment of liver cancer:
1. The level of evidence was reduced from 2A to 2B, and changed from “other recommended options” to “useful options under certain circumstances.”
2. Other recommended options: Nivolumab was removed as a treatment option for Child-Pugh A patients.
The confirmatory study did not meet the primary endpoint: Navulimab regrets its departure from the field of single-drug therapy for liver cancer
In September 2017, the FDA accelerated the approval of the PD-1 inhibitor nivolumab for HCC patients treated with sorafenib based on the results of the Phase I/II CheckMate-040 trial, regardless of PD-L1 status. This is also the first PD-1 approved in the field of liver cancer, opening a precedent for liver cancer immunotherapy.
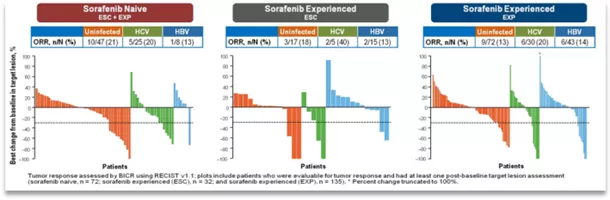
The study is a multi-center, open-label, dose escalation and expansion study. The study enrolled 262 patients with advanced HCC with or without HCV or HBV infection. The dose-escalation period received navuliu 0.1-3mg/Kg treatment, Q2w (ESC, n=48), and the extended period received navuliu 3mg/ Kg treatment, Q2w (EXP, n=214), the primary endpoint of the dose escalation phase is safety and tolerability, and the expansion phase is ORR (Recist1.1 standard assessment).
The results showed that the ORR of all patients was 15%-20%, and the DCR was 58%-64%. The median OS reached 15.6 months. And regardless of whether there is HBV/HCV infection, patients can benefit!
After obtaining accelerated approval, the follow-up Nivolumab continued to conduct a phase III clinical study-CheckMate-459, but unfortunately, the study failed to reach its primary endpoint of overall survival.
The purpose of this study is to evaluate the efficacy of nivolumab and sorafenib head-to-head comparison of first-line treatment of patients with advanced HCC.
The main study endpoint OS did not reach a significant statistical difference (16.4m vs 14.7m HR, 0.85, 95% CI , 0.72-1.02), the ORR was 15% and 7%, respectively, which was consistent with the objective response rate data reported by the CheckMate-040 trial.
Limited monotherapy for advanced liver cancer: The advantages of nivolumab combination therapy are fully demonstrated
1. Adjuvant treatment of early liver cancer: Nivolumab immune monotherapy has shown curative effect
NIVOLVE is a phase II prospective multicenter single-arm trial designed to evaluate the effectiveness and safety of nivolumab as an adjuvant treatment for HCC, and to determine whether surgical resection (SR) or radiofrequency ablation (RFA) predicts patient recurrence Of biomarkers. The study included 53 patients with liver cancer who showed complete remission after SR or RFA.
The results of the study showed that the 1-year RFSR and median RFS were 76.7% and 26.0 months, respectively, and there was no difference between receiving SR and RFA.
The results of exploratory biomarker analysis showed that increased copy number of WNT/b-catenin-related genes (APC, CTNNB1, TCF7L1, TCF7L2) (CNGs) was associated with shorter RFS (positive: 11.8 months vs. negative: not reached[ NR];p=0.0003).
Immunohistochemistry showed that PD-1 was negative (p <0.0001), the PD-L1 combined positive score was low (p = 0.0113), the number of CD8+ tumor infiltrating lymphocytes (TILs) was low (p = 0.0130), and Foxp3+ cells were positive (p = 0.0076) is associated with recurrence. The RFS of ctDNA-positive patients (n = 10) before the use of nivolumab was often shorter than that of ctDNA-negative patients (26.2 vs NR); there was no correlation between TMB and RFS.
In terms of safety, the incidence of grade 3 to 4 treatment-related adverse reactions was 18.9%, and immune-related AEs accounted for 25%.
It can be seen that in the NIVOLVE trial, the 1-year RFSR and RFS were 76.6% and 26.0 months, respectively; the safety was tolerable, and no new adverse reactions were observed. The CNG of WNT/b-catenin-related genes, the activation of WNT/b-catenin pathway, the presence of Foxp3+ cells and the decrease in the number of CD8+TILs may predict the recurrence of SR or RFA patients after adjuvant nivolumab therapy.
2. “O+Y” combined second-line treatment of advanced liver cancer has been included in the CSCO guidelines
“O+Y” has been approved for the second-line treatment of liver cancer patients, and it has been recommended by level III experts in the 2020 CSCO liver cancer guidelines (class 2A evidence). The clinical phase 1/2 study of CheckMate-040 included a total of 148 patients with advanced liver cancer who had previously received sorafenib treatment or were intolerant, and were randomly assigned to three groups: A, B, and C:
Group A (n=50): O drug 1mg/kg + Y drug 3mg/kg, (O1+Y3) once every 3 weeks, follow-up O drug 240mg for 4 consecutive medication cycles, once every 2 weeks;
Group B (n=48): O drug 3mg/kg + Y drug 1mg/kg, (O3+Y1) once every 3 weeks, follow-up O drug 240mg for 4 consecutive medication cycles, once every 2 weeks;
Group C (n=48): O drug 3mg/kg + Y drug 1mg/kg, (O3+Y1) where O drug once every 2 weeks, Y drug once every 6 weeks;
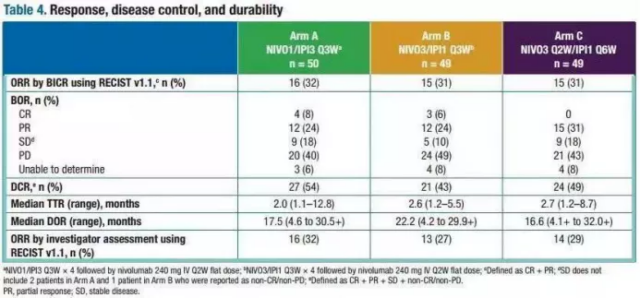
The results of the study showed that the overall patient’s ORR was 33%, of which the CR (complete remission) rate was 8%, and the PR (partial remission) rate was 24%. Regardless of whether the PD-L1 expression level is high or low, there are those who see benefit . The specific effects of each group are as follows:
- Group A: ORR was 32%, CR was 8%, PR was 24%, and OS was 22.8 months;
- Group B: ORR was 31%, CR was 6%, PR was 24%, OS was 12.5 months;
- Group C: ORR was 31%, CR was 0%, PR was 31%, and OS was 12.7 months.
3. A number of studies are underway, and nivolumab is expected to “recover” liver cancer indications!
Phase I/II, dose escalation, open-label, non-comparative study of nivolumumab or nivolumumab combined with ipilimumab in the treatment of patients with advanced liver cancer with or without chronic viral hepatitis; A randomized, open-label study of monoclonal antibodies versus sorafenib in patients with advanced liver cancer who did not receive systemic therapy.

A phase III, randomized, double-blind, nivolumab adjuvant treatment and placebo-controlled study for patients with liver cancer at high risk of recurrence after therapeutic hepatectomy or ablation (CheckMate-9DX study).

It can be seen that although the FDA’s Oncology Drug Advisory Committee opposed the continued accelerated approval of nivolumab with 5 to 4 votes, Bristol-Myers Squibb withdrew the indication for liver cancer monotherapy for advanced liver cancer, but Nivolumab’s research on liver cancer has not stopped.
Whether it is a single agent or a combination, early adjuvant or advanced system therapy is underway, we look forward to the early return of Nivolumab to the field of liver cancer!
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



