Targeting STING Cancer immunotherapy
- A Single US$2.15-Million Injection to Block 90% of Cancer Cell Formation
- WIV: Prevention of New Disease X and Investigation of the Origin of COVID-19
- Why Botulinum Toxin Reigns as One of the Deadliest Poisons?
- FDA Approves Pfizer’s One-Time Gene Therapy for Hemophilia B: $3.5 Million per Dose
- Aspirin: Study Finds Greater Benefits for These Colorectal Cancer Patients
- Cancer Can Occur Without Genetic Mutations?
Targeting STING Cancer immunotherapy
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Targeting STING Cancer immunotherapy
Over the past decade, cancer immunotherapy using immune checkpoint inhibitors has achieved unprecedented success in cancer treatment;
however, only a small fraction ( 10-35% ) of patients can derive clinical benefit from this treatment, Therefore, there is an urgent need to identify new strategies to improve the clinical efficacy of immune checkpoint inhibitors.
Recently, accumulating clinical evidence has confirmed that the effectiveness of ICIs cancer immunotherapy depends on the pre-existing anti-tumor T cell response in the tumor, and T cell infiltration in the tumor is positively correlated with the prognosis of immunotherapy.
However, the initiation and maintenance of T cell responses is dependent on the innate immune response, which is also a key factor in anticancer immunity.
As an innate cytoplasmic DNA sensing pathway, the cyclic GMP-AMP synthetase ( cGAS )-stimulator of interferon genes ( STING ) signaling pathway has attracted much attention for its ability to activate innate and adaptive immunity, which Helps host defense against cancer.
Based on the critical role of the cGAS-STING signaling pathway in anticancer immunity, various STING agonists have been developed and proved to be a promising cancer treatment strategy.
cGAS-STING signaling pathway
The innate immune system can recognize pathogens or injury-associated patterns through pattern recognition receptors, including cGAS. cGAS is an innate immune sensor of cytoplasmic double-stranded DNA ( dsDNA ), with a catalytic domain and two major DNA-binding sites.
Cytoplasmic DNA, whether exogenous or endogenous, can bind in a sequence-independent manner through interactions between its phosphate backbone and the positively charged sites of cGAS to form a 2:2 complex.
After binding to DNA, cGAS undergoes substantial conformational changes in the catalytic pocket, which facilitates the activation of cGAS, thereby enhancing the synthesis of the second messenger cGAMP from adenosine triphosphate ( ATP ) and guanosine triphosphate ( GTP ).
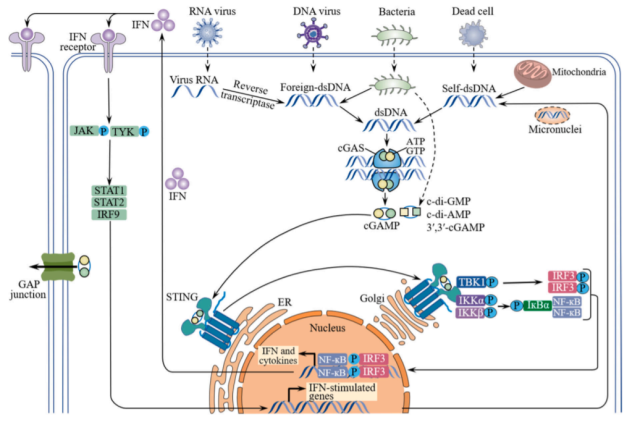
Upon binding to cGAMP, the endoplasmic reticulum ( ER )-bound STING dimer undergoes a substantial conformational transition from an inactive open form to an active closed form and triggers its translocation from the endoplasmic reticulum to the Golgi apparatus, where STING is recruited Phosphorylation of Tank-binding kinase 1 ( TBK1 ) leads to the recruitment of interferon regulatory factor 3 ( IRF3 ).
After being phosphorylated by TBK1, IRF3 dimerizes and enters the nucleus to drive the expression of type I interferons. Newly expressed type I interferons act in an autocrine and paracrine manner by binding to the heterodimeric receptor IFNAR1/2, which then activates Janus kinase 1 (JAK1) and tyrosine kinase 2 ( TYK2 ), leading to signaling Phosphorylation of transducer and activator of transcription 1 ( STAT1 ) and STAT2.
STAT1/2 heterodimers bind to IRF9 and subsequently translocate to the nucleus, triggering the transcription of IFN-stimulated genes ( ISGs ). To some extent, STING also recruits IκB kinase ( IKK ), which phosphorylates the nuclear factor-κB ( NF-κB ) inhibitor IκBα, leading to translocation of NF-κB to the nucleus and subsequent expression of pro-inflammatory cytokines such as IL-6 , TNF, etc. ).
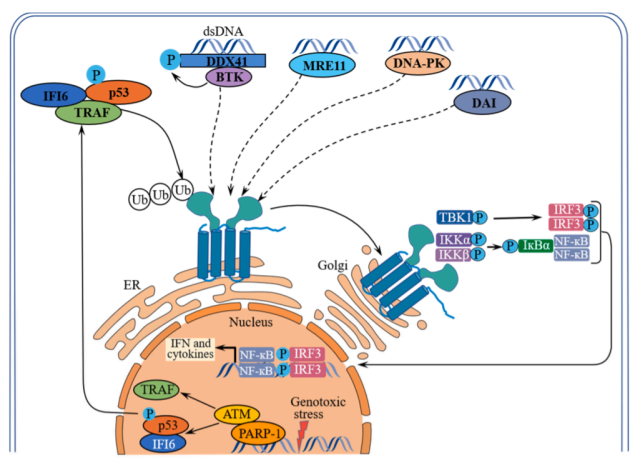
In addition to cGAS, activation of the STING signaling pathway can also be mediated by other DNA sensors, including interferon gamma-inducible protein 16 ( IFI16 ), DEAD box helicase 41 ( DDX41 ), meiotic recombination 11 homolog A ( MRE11 ), DNA-dependent protein kinase ( DNA-PK ) and DNA-dependent IRF activator ( DAI ).
The role of STING activation in anticancer immunity
The cGAS-STING signaling pathway has been shown to be involved in different stages of the cancer immune cycle, and activation of this cytoplasmic DNA sensing pathway can promote the cancer immune cycle in multiple ways.
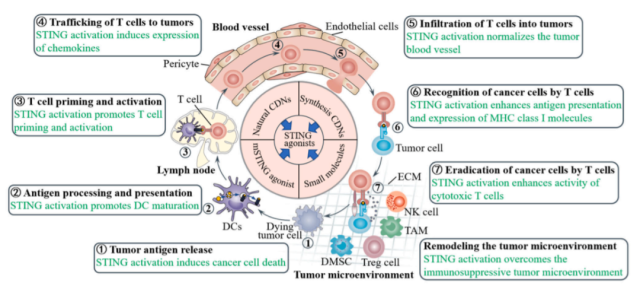
STING activation induces cancer cell death
The intrinsic cGAS-STING signaling pathway of cancer cells is important for anticancer immunity because cytoplasmic DNA accumulation in cancer cells promotes IFN-β production in a cGAS-STI-dependent manner, thereby inhibiting tumor growth and enhancing tumor resistance to checkpoint blockade.
Sensitivity to checkpoint blockade, whereas loss of intrinsic cGAS-STING signaling in cancer cells leads to decreased T cell infiltration and increased resistance to checkpoint blockade. The cGAS-STING signaling pathway has been found to be suppressed in various cancers, and the downregulation of cancer cell-intrinsic cGAS/STING expression has also been shown to correlate with poor clinical outcomes in cancer patients.
Activation of STING in cancer cells contributes to cancer cell death and induction of T cell responses.
STING activation enhances antigen processing and presentation
Tumor cell-derived DNA in the tumor microenvironment can also be taken up by DCs through phagocytosis, which subsequently activates the cGAS-STING pathway, promoting the production of type I IFNs that enhance DC maturation and mediate T cell activation.
STING activation promotes T cell priming and activation
T cells are generally considered to be the main players in anti-tumor immunity. However, three signals are required for T cell priming and activation, including tumor antigens on the major histocompatibility complex ( signal 1 ), co-stimulatory molecules ( signal 2 ), and certain proinflammatory cytokines ( signal 3 ). All of these signals can be enhanced by activating the STING pathway.
STING activation promotes homing and infiltration of T cells into tumors
Extravasation and migration of T cells from the vasculature into the tumor microenvironment is a prerequisite for T cell recognition and subsequent elimination of tumor cells. Activation of the STING pathway promotes T cell trafficking and infiltration into tumors in multiple ways.
First, STING pathway activation in DCs promotes the expression of the chemokines CXCL9 and CXCL10, both of which are essential for T cell trafficking into tumors, in a STING-dependent manner.
In addition, activation of STING in tumor endothelial cells upregulates adhesion molecules on endothelial cells, such as E-selectin, vascular cell adhesion protein 1 ( VCAM-1 ), and integrin ligand intercellular adhesion molecule 1 ( ICAM-1 ), enhancing extravasation of T cells from the vasculature.
STING activation enhances T cell recognition and eradication of cancer cells
Activation of STING in cancer cells can upregulate MHC class I molecules on cancer cells and improve tumor antigen presentation, thereby facilitating their recognition and elimination by cytotoxic T cells.
Conversely, defects in the STING signaling pathway shield cancer cells from immune recognition by cytotoxic T lymphocytes and limit their susceptibility to cytotoxic T cell lysis. Furthermore, activation of STING with agonists enhanced the infiltration and activity of cytotoxic T cells in tumors, helping to induce tumor regression.
STING activation remodels the immunosuppressive tumor microenvironment
Immunosuppressive tumor-associated immune cells, including tumor-associated macrophages, myeloid-derived suppressor cells ( MDSCs ), and regulatory T cells, have been shown to directly or indirectly suppress T cell-mediated immune responses.
Indeed, activation of STING not only polarizes tumor-associated macrophages toward antitumor M1 macrophages, but also reprograms M2 macrophages into M1 macrophages. In addition, STING activation inhibits the expansion and T cell suppression of MDSCs, while inducing MDSCs to produce myeloid-homing chemokines, thereby inhibiting the immunosuppressive function of MDSCs and directing a large number of immune cells such as monocytes, macrophages, and T cells Recruited to the tumor site. STING agonists also reduced the levels of regulatory T cells in the tumor microenvironment.
Development of STING agonists
The critical role of STING signaling in antitumor immune responses makes STING an attractive therapeutic target for cancer immunotherapy.
As a result, there has been renewed interest in developing STING agonists both in academia and the pharmaceutical industry. Currently, many STING agonists with preclinical benefits have been reported , some of which have entered clinical trials.
Natural CDN STING Agonist
As the molecular mechanism of the cGAS-STING signaling pathway becomes clearer, the development of STING agonists has been focused on cyclic dinucleotides ( CDNs ), which are natural ligands of STING, such as c- diGMP, c-diAMP, 3′,3′-cGAMP and 2′,3′-cGAMP.
Natural CDNs have shown promise as therapeutic vaccine adjuvants and immunotherapeutics in preclinical studies.
However, clinical development of native CDNs is limited by rapid hydrolysis by phosphodiesterases, poor cytoplasmic delivery, and lack of ability to activate all human STING isoforms.
Synthetic CDN STING agonist
Due to the shortcomings of natural CDNs in anticancer therapy, efforts have been initiated to develop CDN-based synthetic agonists of STING to improve their stability and therapeutic efficacy.
Among them, ADU-S100 ( MIW815 ) has a higher affinity for mouse and human STING than unmodified CDNs. After intratumoral injection, ADU-S100 not only efficiently eliminated multiple tumor types, but also induced robust CD8+ T cell responses.
However, in a Phase I study ( NCT02675439 ) in patients with advanced/metastatic cancer , ADU-S100 had limited clinical activity as a monotherapy, although ADU-S100 was shown to be well tolerated and trigger systemic immune activation .
In addition to ADU-S100, other CDN-based STING agonists were also designed.
MK-1454 is a novel modified CDN developed by Merck. In vitro, MK-1454 exhibited high affinity for STING and showed a strong ability to induce IFN-β secretion. Furthermore, intratumoral injection of MK-1454 induced complete tumor regression and enhanced the antitumor effect of anti-PD1 therapy in mouse syngeneic tumor models.
An ongoing clinical trial is evaluating the safety and efficacy of MK-1454 in combination with pembrolizumab in patients with advanced solid tumors or lymphoma ( NCT03010176 ). Preliminary data suggest that the combination of MK-1454 and pembrolizumab produced encouraging efficacy despite the ineffectiveness of MK-1454 monotherapy.
Based on these data, a phase II clinical trial ( NCT04220866 ) of MK-1454 combined with pembrolizumab in patients with head and neck squamous cell carcinoma is being explored, and the clinical results have not yet been published.
In addition, other CDN-based STING agonists, including S B11285, BI1387446 , and IMSA101 , are also undergoing clinical evaluation.
Small molecule STING agonists
Compared with CDN-based STING agonists, small molecule STING agonists have some obvious advantages, such as superior pharmacokinetics and simple chemical synthesis. Therefore, the focus of STING agonist development has shifted to non-CDN-derived small molecule STING agonists.
Recently, scientists at GlaxoSmithKline identified a series of aminobenzimidazole ( ABZI )-based derivatives as STING agonists through high-throughput screening.
They are 400-fold more potent than cGAMP in STING activation in human peripheral blood mononuclear cells ( PBMCs ) and have also shown potent STING activation in mouse PBMCs. Intravenous administration significantly inhibited tumor growth and improved survival in mice with colorectal cancer, with 80 percent of treated mice achieving a tumor-free status by the end of the study.
A recent study by Merck reported that an orally administered benzothiophene STING agonist named MSA-2 triggered the phosphorylation of TBK1 and IRF-3 and induced THP in human monocytes in a STING-dependent manner. Induced secretion of IFN-β in -1 cells.
Due to its low pKa, MSA-2 exhibited higher cell permeability and potency than normal tissues in the acidic tumor microenvironment, which indicated that MSA-2 can selectively target tumor tissues and thus is suitable for systemic administration, which is relatively Significant advantages over CDNs.
MSA-2 showed dose-dependent antitumor activity in mice bearing MC38 syngeneic tumors, and complete tumor regression was observed in 80%–100% of treated mice.
In addition to the small molecule STING agonists mentioned above, there are several undisclosed small molecule STING agonists in clinical trials. Among them, SNX281 is a new STING agonist with good pharmacokinetic properties.
SNX281 combined with anti-PD-1 antibody showed significant antitumor activity and survival benefit in tumor models including MC38, CT26 and B16-F10. Currently, a phase I trial of pembrolizumab as a single agent and in combination with pembrolizumab is ongoing in advanced solid tumors and lymphomas ( NCT04609579 ).
Additionally, other STING agonists such as TAK-676 ( NCT04420884 ), GSK3745417 ( NCT05424380 ) and MK-2118 ( NCT03249792 ) are also being evaluated in Phase I trials as monotherapy as well as in combination with anti-PD-1 antibodies.
Challenges of Targeting STING
Although the therapeutic potential of STING agonists has been demonstrated in preclinical and clinical studies, the development of STING agonists is still in its infancy and faces serious challenges that hinder its clinical development.
First, STING activation is a double-edged sword in cancer immunity.
Activation of STING not only promotes T cell priming and activation, but activation of STING has also been shown to induce the expression of immunosuppressive molecules such as PD-L1 and IDO.
Surprisingly, long-term exposure to high doses of STING agonists was found to induce T cell apoptosis, which is exactly the opposite of the expected effect of agonists, whereas low doses of STING agonists elicited robust T cell responses and correlated with Immune checkpoint inhibitors act synergistically.
Therefore, in order to obtain the best antitumor effect of STING agonists, it is necessary to optimize the dose and administration time of STING agonists to avoid overstimulation and negative regulation.
Second, due to single nucleotide polymorphisms in the STING gene, there are five major types of STING variants in humans, including R232 ( 57.9% ), HAQ ( 20.4% ), R232H ( 13.7% ), AQ ( 5.2% ), and R293Q ( 1.5% ). These human STING variants have been shown to be differentially responsive to STING agonists.
In addition, the distribution of these variants varies among different ethnic groups, for example, the HAQ variant is common in East Asians but rare in Africans. Therefore, the biased distribution of STING variants in the population should be considered when recruiting patients for clinical trials.
Overall, human STING genes have a large heterogeneity and biased distribution in the population, which poses a barrier to the development of STING agonists.
Third, STING is widely expressed in many cell types, including cancer cells and non-cancer cells. Currently, although STING agonists suitable for systemic administration have been developed, almost all agonists lack tumor specificity ( except MSA-2 ).
Therefore, systemic administration of STING agonists has the potential to indiscriminately activate STING systemically, thereby inducing the secretion of pro-inflammatory cytokines in tumor and non-tumor tissues, and even triggering a cytokine storm.
Therefore, systemically administered STING agonists should specifically target tumor tissues and elicit antitumor immune responses in the tumor microenvironment, thereby maximizing antitumor efficacy and mitigating side effects against normal tissues.
Summary
In summary, the cGAS-STING signaling pathway is an important innate immune pathway that plays a crucial role in cancer immune surveillance.
STING activation has been shown to promote the cancer immune cycle and transform the immunosuppressive tumor microenvironment into an immune-supportive microenvironment.
Due to the immunostimulatory effect of STING , a variety of STING agonists have been developed in recent years , and many are currently in clinical trials.
However, the development of STING agonists still faces many challenges, including the dual role of STING signaling, and the heterogeneity and ubiquitous expression of STING. In addition, our understanding of the cGAS-STING signaling pathway still needs to be improved.
For example, the mechanisms underlying cGAS-STING signaling suppression in cancer are currently unclear. Likewise, how STING activation induces the expression of immunosuppressive molecules, including PD-L1 and IDO, has not been elucidated.
These challenges and unknowns will help drive continued research on this pathway, and new discoveries in this field will allow STING agonists to play a greater role in cancer immunotherapy.
references:
1. Targeting STING for cancer immunotherapy: From mechanisms to translation. Int Immunopharmacol.2022 Dec;113(Pt A):109304
Targeting STING Cancer immunotherapy
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



