Why is Metalloproteases important in cancer immunotherapy?
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Why is Metalloproteases important in cancer immunotherapy?
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Why is Metalloproteases important in cancer immunotherapy?
Metalloproteases ( MPs ) are a large family of proteases with metal ions in their active centers.
According to different structural domains, metalloproteases can be divided into various subtypes, mainly including matrix metalloproteinases ( MMPs ), disintegrin metalloproteases ( ADAMs ), and ADAMs with thrombospondin motifs ( ADAMTS ).
They have diverse functions such as proteolysis, cell adhesion, and extracellular matrix remodeling.
Metalloproteases are expressed in various types of cancers and participate in many pathological processes involving tumorigenesis, progression, invasion, and metastasis by regulating signal transduction and the tumor microenvironment.
Therefore, a better understanding of the expression patterns and functions of MPs in cancer immune regulation will facilitate the development of more effective cancer diagnostics and immunotherapies.
MP structure and expression
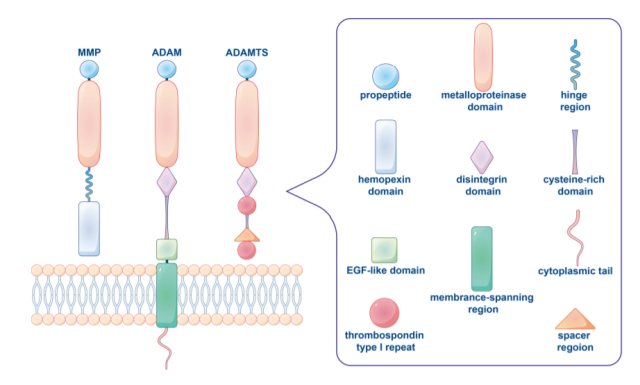
Matrix Metalloproteinases (MMPs)
In vertebrates, the MMP family consists of 28 members, at least 23 of which are expressed in human tissues, 14 of which are expressed in the vasculature. Matrix metalloproteinases are usually divided into collagenases ( MMP1, MMP8, MMP13 ), gelatinases ( MMP2, MMP9 ), hemolysins ( MMP3, MMP10, MMP11 ), stromelysins ( MMP7) according to their substrates and the organization of their domains. , MMP26 ), membrane MMPs ( MT MMPs ) or other MMPs. The MMP family has a common core structure.
Typical MMPs consist of a propeptide of approximately 80 amino acids, a metalloprotease catalytic domain of 170 amino acids, a linker peptide or hinge region of variable length, and a heme protein domain of approximately 200 amino acids.
Different types of MMPs have specific structural features that differ from typical MMPs. For example, MT MMPs lack the prodomain, while MMP7, MMP26, and MMP23 lack the Hpx domain and linker peptide. Furthermore, MMP2 and MMP9 contain three repeats of fibronectin.
These distinct domains, modules, and motifs in MMPs are involved in interactions with other molecules, thereby affecting or determining MMP activity, substrate specificity, and cellular and tissue localization.
MMPs have been detected in a variety of human cancers, and high expression of MMPs is generally associated with reduced survival in most cancers, including colorectal, lung, breast, ovarian, and gastric cancers.
Among them, MMP2 and MMP9, capable of degrading type IV collagen in basement membrane, are the most widely studied metalloproteases and are associated with disease progression and reduced survival in patients with various cancers.
Disintegrin metalloprotease (ADAM)
ADAMs are type I transmembrane proteins anchored on the cell surface membrane, and more than 30 kinds have been discovered so far. Like MMPs, ADAMs include a prodomain and a zinc-binding metalloprotease domain. ADAM also includes a disintegrin domain unique among cell surface proteins.
The metalloprotease domains of ADAMs are highly conserved, with most ADAMs having a cysteine-rich domain and an EGF-like domain adjacent to the transmembrane region, followed by a length and sequence that vary between different ADAM family members Large intracellular domain.
Due to the presence of these domains, ADAMs can bind substrates and affect changes in cell adhesion and migration, as well as the proteolytic release of cell surface molecules. Their major substrates are intact transmembrane proteins such as precursor forms of growth factors, adhesion molecules and cytokines.
Cancer cells often express high levels of ADAMs, and ADAM17 is the most extensively studied of all ADAM proteins.
A study evaluating ADAM17 as a potential blood biomarker for ovarian cancer demonstrated significantly higher levels of ADAM17 in culture supernatants of ovarian cancer cell lines as well as in serum and ascites of ovarian cancer patients compared with controls.
ADAMs with thrombospondin motifs (ADAMTS)
Unlike ADAM, ADAMTS is a secreted metalloprotease characterized by an accessory domain containing thrombospondin type 1 repeat ( TSR ) and spacer, and lacking transmembrane, intracellular and ( EGF )-like domains, The human ADAMTS family includes 19 proteins.
ADAMTS proteases are involved in the maturation of procollagen and von Willebrand factors, as well as in ECM proteolysis associated with morphogenesis, angiogenesis, and cancer.
Studies have shown that different ADAMTS have different biological functions, and individual ADAMTS can play different roles in different cancers or according to the clinical setting. Compared with MMPs and ADAMs, the involvement of ADAMTS in TME has been less studied, thus there is an urgent need to systematically study their functions in cancer.
Signaling pathways involving immune-associated MPs in cancer cells
Signal transduction pathways consist of multiple molecules that recognize and interact with each other and transmit signals to regulate many important biological processes, such as tumor cell proliferation, metastasis, and immune regulation. Three signaling pathways are especially closely related to MP in immune regulation .
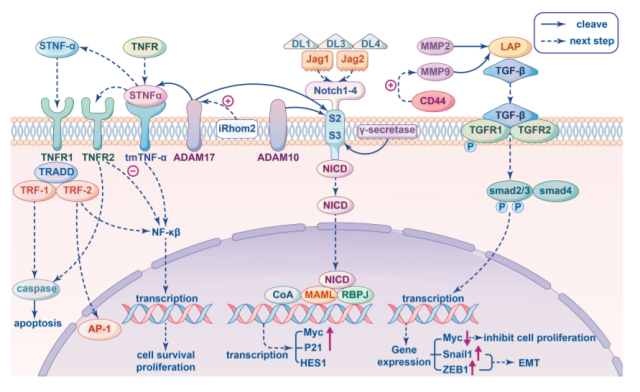
TNF signaling
Tumor necrosis factor-alpha ( TNF-α ) is an important pro-inflammatory cytokine involved in the maintenance and homeostasis of the immune system, as well as inflammation and host defense.
Soluble TNF-α is cleaved from transmembrane TNF-α ( tmTNF-α ) by the proteolytic enzyme ADAM17, also known as TNF-α converting enzyme ( TACE ), which can coordinate immune and inflammatory responses by activating TNF-α. Given the role of ADAM17 on receptors and ligands of the TNF signaling pathway, ADAM17 is thought to affect TNF-α signaling in multiple ways.
For example, a reduction in soluble TNF-α production will lead to accumulation of tmTNF-α, which will bind to TNFR2 and lead to different biological outcomes.
Transforming growth factor-beta
Transforming growth factor-β ( TGF-β ), as a key regulator of tumor behavior, plays an important role in tumor invasion and metastasis, immune regulation and therapy resistance. TGF-β is also central to TME immunosuppression, with pleiotropic functions on the immune system depending on the context.
MMP9 and MMP2 are two metalloproteases known to cleave inactive TGF-β precursors and generate distinct TGF-β proteolytic cleavage products, resulting in TGF-β activation. Furthermore, degradation of fibronectin by MMP9 bound to CD44 leads to the release of active TGF-β.
The level of MMP9 in cancer cells may not only affect the proteolysis of TGF-β, but also affect the expression of TGFβ and downstream substances of TGF signaling pathway.
Studies on the relationship between MMP9 and TGF signaling pathways in breast cancer have shown that the overexpression of MMP9 in breast cancer cells not only significantly up-regulates the expression of SMAD2, SMAD3 and SMAD4, but also enhances the phosphorylation of SMAD2.
Notch signaling pathway
Notch signaling is involved in multiple aspects of tumor biology, and its role in the development and regulation of the immune response is complex, including shaping the immune system and components of the TME, such as antigen-presenting cells, T cell subsets, and communication between cancer cells. complex crosstalk. In particular, Notch plays a key role in the development and maintenance of different immune cells.
After ligand binding to Notch receptors, downstream signaling is mediated by several proteases including members of the ADAM family.
First, the receptor/ligand interaction exposes the proteolytic cleavage site S2, which is cleaved by ADAM metalloproteases.
γ-Secretase-mediated subsequent cleavage at S3 occurs at the transmembrane region, leading to the release of the Notch intracellular domain ( NICD ), which translocates into the nucleus and binds MAML to RBPJ, triggering target genes such as Myc , P21 and HES1 transcription.
ADAM10 and ADAM17 are known to be involved in the cleavage of S2, with ADAM17 leading to ligand-independent Notch activation and ADAM10 leading to ligand-dependent activation.
Regulation of the tumor microenvironment by MPs
TME refers to the microenvironment around tumor cells, including blood vessels, immune cells, fibroblasts, myeloid-derived suppressor cells, various signaling molecules, and the ECM . The TME plays a key role in regulating the immune response to cancer.
Effect of MP on ECM
The ECM is an acellular component of the TME matrix, and remodeling of the ECM plays an important role in cancer development and homeostasis, as well as immune cell recruitment and tissue metastasis. Extensive remodeling of the ECM during cancer progression leads to changes in its density and composition, specifically, protease-induced breakdown of ECM components is critical for tumor cells to cross tissue barriers.
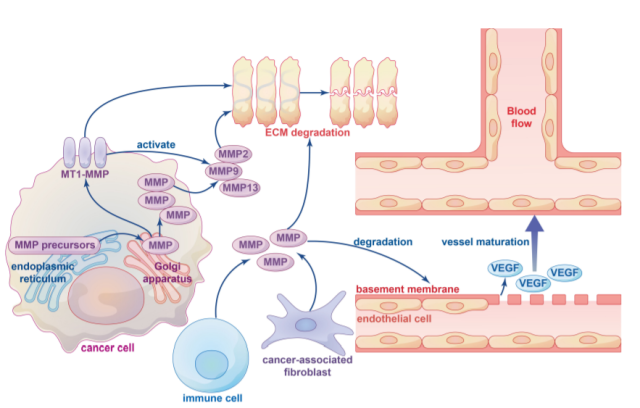
MMPs and ADAMs are the main enzymes involved in ECM degradation, and MMPs involved in ECM degradation can be roughly divided into membrane-anchored MMPs and soluble MMPs.
ECM degradation is mainly achieved by soluble MMPs ( such as MMP2, MMP9, and MMP13 ) activated by MT1 MMP. ECM has three main components: fiber, proteoglycans, and polysaccharides.
MMPs play an important role in tissue remodeling by binding to these matrices to facilitate the turnover of various ECM proteins. The specific mechanism by which MMPs degrade ECM is unclear and needs further study.
The relationship between MP and immune cells
MP plays an important role in promoting immune cell activity and regulating immune cell migration. The relationship between MP and immune cells is shown in the figure below.
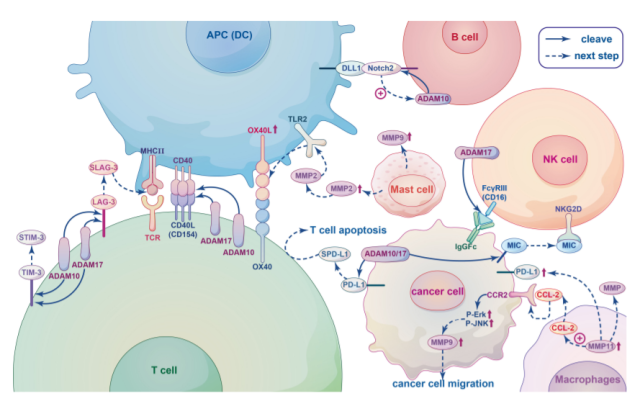
ADAM10 and ADAM17 are expressed on the surface of quiescent CD4+ Th cells and are important for regulating CD4+ Th development and function.
ADAM10/17 plays a key role in the shedding of T cell co-stimulatory receptors as well as co-inhibitory receptors. For example, CD154 ( CD40L ), a type II membrane co-stimulatory receptor, is rapidly upregulated within a few hours following the interaction between T cells and APCs, followed by subsequent cleavage from the T cell surface by ADAM10 and ADAM17. freed.
In addition, ADAM10 and ADAM17 also act on the co-stimulatory receptor CD137, and the inhibitory receptors LAG-3, TIM-3, soluble forms of sLAG-3 and sTIM-3 are formed after proteolytic cleavage of ADAM10 and ADAM17.
B cells are a key cellular component of humoral immunity, and marginal zone B cells ( MZB ) located in the spleen express high levels of CD80/86 co-stimulatory molecules, leading to T cell activation. Notch2 signaling is required for MZB cell development.
During MZB development, Notch2 heterodimers bind to ligands such as DLL1 on stromal cells and APCs, which initiates an unknown metalloprotease hydrolytic receptor, leading to Release of the Notch intracellular domain, which translocates to the nucleus and triggers the expression of downstream target genes. This unknown metalloprotease may be ADAM10.
NK cells express the IgG Fc receptor FcγRIII ( CD16 ), CD16 molecules can be cleaved from the surface of activated NK cells by ADAM17, inhibition of ADAM17 will weaken the extracellular shedding of CD16 and CD62L, thereby significantly increasing intracellular TNF-α and IFN-γ s level.
In addition, MMPs and ADAMS can cleave ligands for the activating receptor NKG2D from the surface of tumor cells. Soluble forms of these cleavage proteins bind NKG2D and induce endocytosis and degradation of this receptor, leading to tumor evasion from surveillance.
Collectively, multiple substrates for ADAM17 cleavage are associated with distinct roles in NK cells.
Tumor-associated macrophages ( TAMs ) contribute to cancer initiation and malignant progression, and high levels of TAMs are associated with poor prognosis and reduced overall survival.
In a variety of cancers, TAMs have been found to promote tumor angiogenesis and invasion and regulate immune responses by secreting MMPs. The regulation of MMPs is closely related to the chemokines secreted by TAMs.
Immunomodulatory cytokines associated with MPs
A variety of tumor cell-derived cytokines, including TGF-β, EGF, HGF, and TNF-α, mediate the expression of many MPs. The most important of these is MMP9, which is elevated in serum and tumor-associated tissues and is involved in the degradation of the ECM to promote the migration of immune cells in cancer.
Furthermore, these cytokines must be cleaved by MPs to participate in the tumor immune process. For example, TmTNF-α cleaved by ADAM17 produces active sTNF-α. IL-12 also plays a key role in T cell development and expansion, and inactive IL-12 precursors need to be converted to an active state in the TME after cleavage by MMP14.
Metalloproteases and angiogenesis
To date, several types of tumor angiogenesis have been reported, including sprouting angiogenesis and angiogenesis mimicry ( VM ). Sprout angiogenesis is achieved through the upregulation of various hydrolytic enzymes ( such as MP and tissue plasmin ) in the vascular basement membrane, which leads to the degradation and remodeling of the basement membrane and ECM. For example, in pancreatic neuroendocrine tumors, increased MMP9 secretion releases sequestered VEGF from the stroma, thereby converting vasculature quiescence to active angiogenesis. In lung cancer cells, inhibition of MMP2 activity reduced its interaction with the integrin AVB3 and inhibited the expression of VEGF mediated by downstream PI3K/AKT signaling, resulting in reduced angiogenesis.
VM is a new model in which aggressive tumors form new blood vessels to provide blood supply for tumor growth. Studies have shown that the initial hypoxic environment of solid tumors is inseparable from VM, and hypoxia is closely related to the expression and activity of MMPs. Hypoxia-inducible factor-1α ( HIF-1α ) has been shown to directly regulate the expression of MMP14, MMP9, and MMP2.
Immunotherapy targeting MP
In view of the role of MP in cancer immune regulation, people began to explore immunotherapy targeting MP , and a variety of broad-spectrum MP inhibitors appeared in clinical trials .
However, MP inhibitors have so far failed to improve the survival and prognosis of cancer patients due to the nonspecific targeting of drugs and the complex role of MPs in immune regulation .
Recently, it has been reported that MP inhibitors can be used in combination therapy to improve the efficacy of immunotherapy.
SB-3CT, as an MMP2/9 inhibitor, is believed to enhance the efficacy of anti-PD-1 and anti-CTLA-4 therapy in mouse models of melanoma and lung cancer.
SB-3CT treatment not only resulted in decreased PD-L1 expression by reducing multiple oncogenic pathways, but also significantly improved immune cell infiltration and T cell cytotoxicity when combined with anti-PD-1 therapy.
Furthermore, the combination of SB-3CT with anti-CTLA-4 enhanced the downregulation of PD-L1 expression and increased the abundance of activated tumor-infiltrating CD8+ T cells in tumors.
Andecaliximab ( GS-5745 ) is a monoclonal antibody that selectively inhibits MMP9. GS-5745 inhibits MMP9 by binding to MMP9 precursor and preventing MMP9 activation, while binding to active MMP9 inhibits its activity. Fab 3369 targets MMP14, blocks endogenous MMP14 expressed on the cell surface, and inhibits ECM degradation in triple-negative breast cancer ( TNBC ).
In addition, there are several antibodies that effectively inhibit ADAM17, including A12, A9, and MED13622. There are also small molecule inhibitors in clinical development that have shown positive effects in clinical trials.
Summary
MPs play important roles in immune regulation in the TME, including ECM remodeling, signaling pathway transduction, cytokine shedding and release, and promotion of angiogenesis. Emerging technologies and drugs related to MP are increasingly explored in cancer diagnosis and therapy.
Therefore, a better understanding of the expression patterns and functions of MPs in cancer immune regulation will facilitate the development of more effective cancer diagnostics and immunotherapies.
MP-based exploration and new technologies have great potential, and they may provide effective strategies for future cancer diagnosis and treatment.
references:
1.Immunomodulatory role of metalloproteases in cancers: Current progress and future trends. Front Immunol.2022; 13: 1064033.
Why is Metalloproteases important in cancer immunotherapy?
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



