The world first clinical phase III study of adjuvant immunotherapy for liver cancer is successful
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
The world first clinical phase III study of adjuvant immunotherapy for liver cancer is successful
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
The world first clinical phase III study of adjuvant immunotherapy for liver cancer is successful, and the “T+A” program will bring new life to many patients.
At the just-held annual meeting of the American Association for Cancer Research (AACR), the highly anticipated phase III clinical study of hepatocellular carcinoma (HCC) – IMbrave050 announced the results of the interim analysis for the first time.
The immune + anti-vascular combination therapy regimen (T+A regimen) composed of valizumab can significantly reduce the risk of recurrence, distant metastasis or death of HCC patients by 28% after radical treatment (surgical resection or ablation) [ 1 ]!

IMbrave050 research results presented at the AACR annual meeting
This is the first randomized controlled, multi-center, clinical phase III study reporting positive results in the field of HCC adjuvant therapy in the world for many years , and this “first victory” is not easy at all: the HCC that is combated by immune + anti-vascular combined therapy, but relapse The cancer rate is as high as 70%, which has restricted the survival of patients for a long time in the past.
So how did the T+A program deliver excellent answers?
The road of exploration is full of thorns
According to the Global Cancer Statistical Report (GLOBOCAN) released by the World Health Organization in 2020, gloablly more than one million new cases of liver cancer are diagnosed per year and more than 820,000 deaths due to liver cancer.
The academic community also predicts that by 2040, the global incidence and death of liver cancer will increase by 55% compared with 2020.
There are more and more patients, and the disease burden is getting heavier, but the survival rate of liver cancer patients has not improved significantly.
The 5-year survival rate of patients in many countries less 12% [3].
After all, there are one obstacle after another on the road to long-term survival and cure of liver cancer patients.
First, due to the insidious onset and lack of sensitive and convenient early screening methods, early diagnosis of liver cancer is relatively difficult.
According to the survey results, only about 43% of patients with liver cancer are diagnosed at a relatively early stage (AJCC TNM stage I-II) , which may meet the conditions for radical treatment such as surgical resection, ablation or liver transplantation [4].
Early diagnosis is only the first hurdle, radical treatment is the second hurdle, and the extremely high risk of recurrence or metastasis after treatment is the third “deadly” difficulty: a large number of research data have shown that whether it is surgical resection or For ablation therapy, the cumulative recurrence rate of HCC patients within 5 years after radical treatment is as high as 50-70% .
Most of the recurrences are concentrated within 2 years after treatment, but there is also a long-term recurrence risk that cannot be underestimated [5-6].

After curative treatment for HCC, patients still have a high residual risk of recurrence
(Image source: Clinical and Molecular Hepatology )
As we all know, the treatment of recurrent cancer that “comes back” is often difficult. Whether it is re-surgical resection or ablation, the curative effect on recurrent HCC is not good enough. So can effective adjuvant therapy be used to reduce the risk of HCC comeback after the first radical treatment?
At least until the success of the IMbrave050 study, the answer is “no”. Although domestic and foreign academic circles have made a lot of explorations, none of the programs can break through strictly controlled (placebo-controlled, randomized studies) clinical studies, and there are countless failure cases.
Authoritative liver cancer diagnosis and treatment guidelines still have a blank in adjuvant treatment recommendations .
Therefore, the challenge of the T+A plan is undoubtedly an extremely difficult opponent, but many people have long been full of confidence in the T+A plan.
The key weaknesses of the opponent, as well as the previous successful experience, are the keys to defeating the enemy and winning .
Mixed treatments bring more effectivity
Because of the brutality and fatality of HCC, immunotherapy represented by PD-1/L1 inhibitors began to tackle HCC treatment soon after its advent. benefit, so the direction of follow-up exploration has become immunocombined therapy.
In 2020, the key clinical phase III IMbrave150 study of the T+A program was a great success, becoming the first immune combination therapy program that effectively prolongs the survival of patients with advanced HCC, and truly brought HCC into a new era of immunotherapy. From this perspective , IMbrave050 research is also “standing on the shoulders of giants”.
The T+A program was the first to break the situation, and it also benefited from the two drugs of the combined treatment program, which can achieve complementary advantages in the fight against HCC: the mechanism of immunotherapy has been widely known, but the immune microenvironment of HCC is obviously suppressive.
Immunosuppressive cells such as derived immunosuppressive cells (MDSCs), regulatory T cells (Tregs), and many non-cellular components constitute a large network that limits anti-tumor immunity [7].

The role of different types of immune cell subsets in the immune microenvironment of HCC
(Image from: Nature Reviews Clinical Oncology )
Anti-angiogenic targeted drugs such as bevacizumab targeting the VEGF pathway are the key to help immunotherapy get out of trouble .
After entering the era of immunotherapy, preclinical studies have revealed that VEGF is a key immunoregulatory molecule in the immune microenvironment , and suppressive cell subsets such as MDSCs and Tregs will be affected by it. The main rationale for immunotherapy synergy.
Using anti-angiogenic targeted drugs in combination with immunotherapy can also achieve “multiple birds with one stone”: a major feature of HCC is the rich blood supply of the tumor and numerous new blood vessels, but this will make the tumor microenvironment in a hypoxic state, which is conducive to Cancer cells are not conducive to immune cells, and also affect the delivery of therapeutic drugs.
Targeting the VEGF pathway to normalize blood vessels can correct hypoxia and improve drug delivery, which can also help immunotherapy play a better role [7].
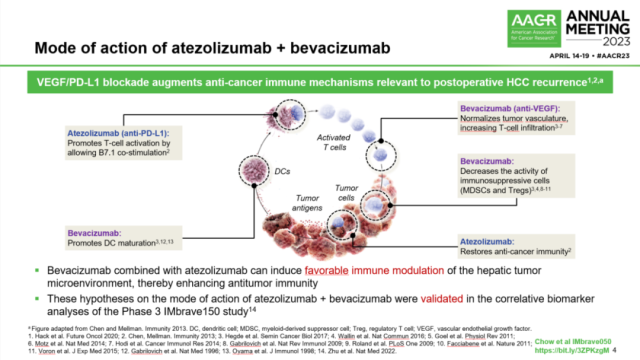
Multiple synergistic mechanisms of T+A regimen combined therapy
With clear mechanism advantages and outstanding performance in the past, a variety of immune + anti-vascular combined therapy programs are advancing towards the cure of HCC, and the T+A program’s IMbrave050 research was the first to succeed, which means that it has become the leader again.
Better Prognosis, More Possibilities
The IMbrave050 study is a typical randomized controlled, multi-center, global clinical phase III pivotal study, and it is dedicated to HCC patients with a high risk of recurrence after radical treatment.
The high-risk characteristics of the surgery group defined by the study include:
1) tumor size > 5cm ;
2) The number of tumors > 3;
3) There is microvascular or large vessel invasion (portal vein invasion is limited to Vp1/Vp2 type);
4) Pathological biopsy shows that the tumor is poorly differentiated (grade 3-4) [8].
A total of 668 patients were randomly assigned to the T+A regimen adjuvant therapy group or the active monitoring group ( The study is open-label), the duration of adjuvant therapy in the T+A program group is about 1 year (17 cycles), and patients in the active monitoring group are allowed to cross over to the T+A program group after disease recurrence (systemic treatment or adjuvant therapy after secondary resection) ).

IMbrave050 study design details
The study used recurrence-free survival (RFS) as the primary endpoint , that is, from randomization to the first occurrence of HCC local/regional recurrence, distant metastasis or death from any cause, which is also the main data announced at the AACR annual meeting; Survival (OS) is one of the secondary endpoints, but as of the publication of the results, the median follow-up time of the IMbrave050 study was 17.4 months, which is not enough to obtain mature OS data.
Then it was time to reveal the key data: the RFS data assessed by the independent review committee (IRF) showed that the 12-month RFS rates of the T+A program group and the active monitoring group were 78% and 65%, respectively, and 12% of the two groups The monthly disease recurrence rates were 20% and 34%, respectively.
The T+A regimen adjuvant therapy significantly reduced the risk of disease recurrence, distant metastasis or death in HCC patients by 28% (HR=0.72), and the study successfully achieved positive results.

IMbrave050 research RFS data Kaplan-Meier curve
According to the recurrence patterns reported in previous studies, the first 2 years after the completion of radical treatment is a relatively concentrated high-risk period for HCC recurrence, which is highly correlated with poor prognosis [ 5,9,10 ].
Improving the 12-month RFS rate of patients is definitely a very good start. The currently observed RFS benefits not only have important clinical significance, but are also very likely to be translated into OS benefits, helping more patients survive long-term and even clinically cure.
In addition, the subgroup analysis results showed that the benefits of each key subgroup of patients were also consistent with the overall population of the study, and the treatment safety of the T+A regimen also met expectations , which was relatively close to the T+A regimen group in the IMbrave150 study.
From the perspective of comprehensive efficacy and safety, the T+A regimen is enough to immediately become the new standard of adjuvant treatment for HCC. It is believed that the authoritative guidelines and consensus at home and abroad will soon be rewritten, and the T+A regimen is strongly recommended.

IMbrave050 Study Adverse Events Occurrence and Comparison with History
It is believed that with the continuous extension of the follow-up period and data updates of the IMbrave050 study, more good news and more comprehensive analysis will also make the use of immune-adjuvant therapy for HCC more precise.
If the T+A plan can open up a broader world for HCC perioperative immunotherapy in the future and allow more patients to receive radical treatment, that would be great But.
references:
[1]Chow P, et al. CT003 – IMbrave050: Phase 3 study of adjuvant atezolizumab + bevacizumab versus active surveillance in patients with hepatocellular carcinoma (HCC) at high risk of disease recurrence following resection or ablation. 3. AACR 202
[2]Rumgay H, Arnold M, Ferlay J, et al. Global burden of primary liver cancer in 2020 and predictions to 2040[J]. Journal of Hepatology, 2022, 77(6): 1598-1606.
[3] Zeng H, Chen W, Zheng R, et al. Changing cancer survival in China during 2003–15: a pooled analysis of 17 population-based cancer registries[J]. The Lancet Global Health, 2018, 6(5) : e555-e567.
[4]Shan T, Ran X, Li H, et al. Disparities in stage at diagnosis for liver cancer in China[J]. Journal of the National Cancer Center, 2023, 3(1): 7-13.
[5]Xu XF, Xing H, Han J, et al. Risk factors, patterns, and outcomes of late recurrence after liver resection for hepatocellular carcinoma: a multicenter study from China[J]. JAMA Surgery, 2019, 154(3) : 209-217.
[6]Kim J, Kang W, Sinn DH, et al. Substantial risk of recurrence even after 5 recurrence-free years in early-stage hepatocellular carcinoma patients[J]. Clinical and Molecular Hepatology, 2020, 26(4): 516 -528.
[7]Llovet JM, Castet F, Heikenwalder M, et al. Immunotherapies for hepatocellular carcinoma[J]. Nature Reviews Clinical Oncology, 2022, 19(3): 151-172.
[8]Hack SP, Spahn J, Chen M, et al. IMbrave 050: a Phase III trial of atezolizumab plus bevacizumab in high-risk hepatitis after curative resection or ablation[J]. Future Oncology, 2020, 16(15) : 975-989.
[9]Yao LQ, Chen ZL, Feng ZH, et al. Clinical features of recurrence after hepatic resection for early-stage hepatocellular carcinoma and long-term survival outcomes of patients with recurrence: a multi-institutional analysis[J]. Annals of Surgical Oncology, 2022, 29(7): 4291-4303.
[10]Wei T, Zhang XF, Bagante F, et al. Early versus late recurrence of hepatocellular carcinoma after surgical resection based on post-recurrence survival: an international multi-institutional analysis[J]. Journal of Gastrointestinal Surgery, 25021, : 125-133.
The world first clinical phase III study of adjuvant immunotherapy for liver cancer is successful
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



