How Do Cancer Cells Metastasize?
- Chinese-made Drug Enters Australia: Causing at Least 20 Deaths!
- UV Rays Threaten Golfers’ Health: Sun Protection Strategies You Need to Know
- RSV: The Hidden Killer of Infants and ToddlersNews draft
- Will Lorlatinib Become A Game-Changer in Treatment for ALK-Positive Lung Cancer?
- Is Nicotine Really Non-Carcinogenic as Tobacco Companies Claim?
- What is the Future of Next-Generation CAR-T Therapy?
How Do Cancer Cells Metastasize?
- Chinese-made Drug Enters Australia: Causing at Least 20 Deaths!
- How serious is Japan’s “flesh-eating bacteria” problem?
- Taiwan 6th wave of COVID outbreak: 623 confirmed cases in one week and 38 deaths
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
How Do Cancer Cells Metastasize?
The five-year survival rate for patients with metastatic cancer is significantly lower than that for those with localized cancer. More than 90% of cancer-related deaths are due to metastasis.
Cancer metastasis is a dynamic and multifaceted process. Normal cells, influenced by biological, physical, and chemical factors, transform into cancer cells.
These cells proliferate uncontrollably, evade the immune system, resist programmed cell death, stimulate angiogenesis, and gain the ability to invade and establish new colonies in distant organs through the bloodstream.
Where Do Cancer Cells Prefer to Metastasize?
Certain primary tumor sites have a higher tendency to metastasize to specific locations.
Let’s delve into the mechanisms behind cancer metastasis with a comprehensive review.
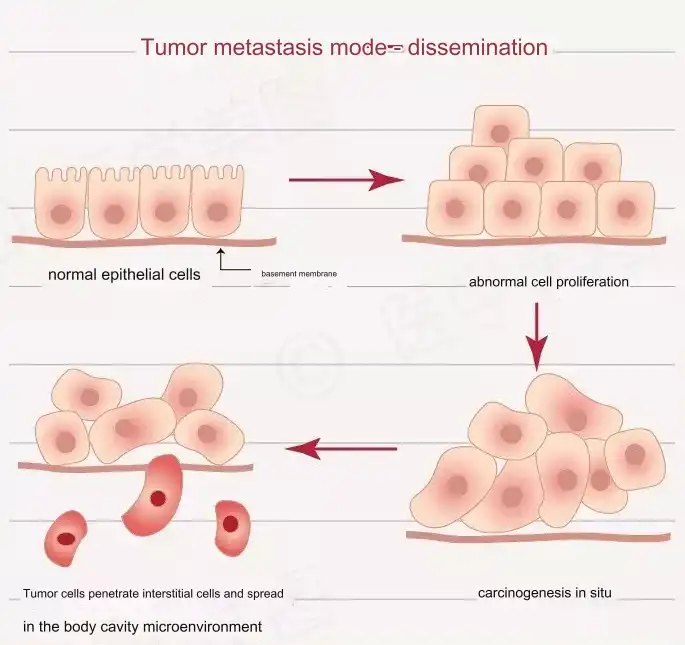
Origin: The Formation of Primary Tumors
The formation of primary tumors is influenced by various factors, including chemical carcinogens, viral infections, epigenetic changes, and somatic mutations. Two models help explain why primary tumors occur.
-
Deterministic Model Hypothesis
This hypothesis suggests that somatic cells can transform into regenerative stem cells through mutations, forming a unique subset of tumor cells with self-renewal capabilities known as cancer stem cells (CSCs). The progeny of these CSCs have limited tumorigenic and metastatic potential, forming the primary tumor.
-
Clonal Evolution Model Hypothesis
According to this hypothesis, mutations and epigenetic modifications give mutated cells (cancer cells) a selective proliferative advantage, leading to uncontrolled cell growth and primary tumor formation. Genetic and epigenetic changes accumulate at different stages of tumor growth, reducing growth suppression.
However, neither hypothesis fully explains the high heterogeneity of primary tumors. The subsequent Cellular Plasticity Model combines these hypotheses, suggesting that normal cells are inherently plastic and can transform into cancer stem cells under internal and external stimuli. This model accounts for the high heterogeneity observed in primary tumors.
Progress: Angiogenesis
It remains unclear when metastasis occurs during primary tumor development. However, it is widely recognized that cancer cells primarily spread through angiogenesis. The abnormal, proliferative, and branched blood vessels in cancerous tissues allow cancer cells to escape from the primary site.
The abnormal vascular system in primary tumors results from an imbalance of angiogenic regulators. Hypoxic and acidic environments in the primary tumor, due to inadequate nutrient and oxygen supply, increase stromal pressure, promoting metastasis.
Angiogenesis can be activated months or even years after primary tumor formation, accelerating tumor growth, continuous replication, and spreading to other areas. Dormant cancer tissues may only appear to be in remission while waiting for angiogenesis to initiate the next phase of metastasis.
In addition to blood vessels, cancer cells can spread through lymphatic or perineural routes and epithelial-mesenchymal transition (EMT). In lymphatic spread, cancer cells disseminate to lymph nodes and distant organs. In perineural spread, cancer cells can travel along nerve fibers. During angiogenesis, not all cancer cells survive entering the vasculature. To metastasize successfully, tumor cells must acquire invasive and stem cell-like characteristics, often by hijacking the EMT developmental program, enhancing their invasive and migratory abilities.
Invasion: Initiating Metastasis
Primary tumor cells can invade blood vessels and migrate to new sites individually or collectively. These invasive cancer cells exhibit specific morphological characteristics, with different molecular mechanisms for each type.
-
Single-Cell Migration
Single-cell migration involves five steps:
- Formation of a leading protrusion through cytoskeletal polarization.
- Attachment of the protrusion to the extracellular matrix (ECM) via cell surface clusters, integrating adhesion and mechanical signaling.
- Activation of surface proteases at the leading edge, degrading ECM components.
- Tension generation by the actomyosin cytoskeleton, causing cell contraction.
- Detachment of adhesion sites at the rear, propelling the cancer cell forward.
The key to single-cell migration is a robust cytoskeleton that withstands migration-related stress to avoid cancer cell death.
-
Collective Migration
Collective migration is considered the primary type of cancer invasion and metastasis. In this process, cancer cells connect through adhesion molecules. Compared to single-cell migration, collective migration has higher invasive and metastatic potential.
Regardless of the migration type, invasion leads to the movement of primary tumors and the entry of cancer cells into the circulation, ultimately causing distant metastasis.
Escape: Evading the Immune System
After entering the bloodstream, cancer cells face challenges before forming secondary tumors. The physical forces in blood vessels (shear stress) and immune surveillance often kill these cells, with less than 0.01% surviving to establish secondary sites.
In circulation, cancer cells resist shear forces by binding to platelets. Additionally, they evade the immune system through various mechanisms, such as releasing soluble factors (VEGF, IL-10, TGF-β, prostaglandin E, and Fas) to create an immunosuppressive environment, downregulating antigen expression, and transferring platelet coatings to themselves to deceive the immune system. These tactics help cancer cells avoid immune detection and eventually establish distant metastases.
Adaptation: Better Suiting the Environment
During primary tumor development, cancer cells continuously alter their metabolism to meet the demands of constant proliferation. Upon metastasizing to secondary sites, cancer cell metabolism changes again. Many cancer cells convert glucose to lactate in the presence of oxygen (Warburg effect), producing less ATP but generating ample subunits for cell growth, thus supporting cancer cell proliferation.
Cancer cells exhibit metabolic plasticity, adapting their surroundings to meet their nutritional needs.
Formation: Large-Scale Metastasis
Once tiny metastatic lesions survive dormancy, grow, and escape immune suppression, cancer cells form large metastases, producing visible tumors over 2 mm in diameter. The “seed and soil” hypothesis by Stephan Paget explains this process. Each cancer cell acts as an organism capable of forming an entire tumor. When cancer cells leave the primary tumor, they adapt to new environments, developing unique traits that allow them to thrive. This heterogeneity explains why cancer cells from different primary sites can metastasize to secondary locations and establish suitable microenvironments.
Understanding the mechanisms of cancer metastasis, from primary tumor formation, angiogenesis, invasion, immune evasion, to environmental adaptation, reveals the complexity and multistage nature of the process. This complexity makes it challenging to block metastasis.
However, this complexity and staging offer multiple therapeutic targets. Treatments targeting different stages of cancer metastasis include:
- Radiotherapy and chemotherapy drugs regulating the cancer cell replication cycle.
- Targeted therapies like metabolic inhibitors, angiogenesis inhibitors, and EMT inhibitors.
- Immunotherapy drugs disrupting the tumor immune microenvironment and enhancing immune response.
- Combination therapies using multiple drug types, showing promising progress in recent clinical studies.
Precision classification of tumor patients and tailored treatments for various pathways will help combat metastatic cancer more effectively, improving five-year survival rates.
How Do Cancer Cells Metastasize?
References:
Maria Castaneda, Petra den Hollander, Nick A. Kuburich, et al. Mechanisms of cancer metastasis. Seminars in Cancer Biology, Volume 87, 2022, Pages 17-31, ISSN 1044-579X, https://doi.org/10.1016/j.semcancer.2022.10.006.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



