Chromosomal Instability Leads to Immune Pathway Rewiring within Cancer Cells
- Paternal Microbiome Perturbations Impact Offspring Fitness
- New Report Casts Doubt on Maradona’s Cause of Death and Rocks Manslaughter Case
- Chinese academician unable to provide the exact source of liver transplants
- Early Biomarker for Multiple Sclerosis Development Identified Years in Advance
- Aspirin Found Ineffective in Improving Recurrence Risk or Survival Rate of Breast Cancer Patients
- Child Products from Aliexpess and Temu Contain Carcinogens 3026x Over Limit
Nature: MSKCC Team Discovers Chromosomal Instability Leads to Immune Pathway Rewiring within Cancer Cells, Resulting in Immune Suppression and Promoting Cancer Metastasis.
- AstraZeneca Admits for the First Time that its COVID Vaccine Has Blood Clot Side Effects
- Was COVID virus leaked from the Chinese WIV lab?
- HIV Cure Research: New Study Links Viral DNA Levels to Spontaneous Control
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Nature: MSKCC Team Discovers Chromosomal Instability Leads to Immune Pathway Rewiring within Cancer Cells, Resulting in Immune Suppression and Promoting Cancer Metastasis.
Under normal circumstances, there is no DNA present in the cytoplasm of cells. However, when chromosome missegregation leads to the leakage of DNA fragments into the cytoplasm, it tricks cells into thinking there is a pathogen invasion. This activation of the cGAS-STING pathway triggers an inflammatory response, leading to the expression of interferon (IFN) to initiate a cell’s intrinsic antiviral or antitumor immune response. It can also recruit T cells to eliminate abnormal cells, helping to suppress carcinogenesis.
Tumors, it appears, are an exception. In 2018, researchers from Memorial Sloan Kettering Cancer Center (MSKCC) and Weill Cornell Medical College published in “Nature” their findings that in tumors with high rates of chromosome missegregation and aneuploidy, sustained activation of the STING pathway not only doesn’t lead to cell death but actually helps tumor cells to spread.
This was quite perplexing.
So, researchers from both institutions collaborated again to answer this question, revealing a new mechanism by which chromosomal instability (CIN) drives tumor metastasis. Their findings were recently published in “Nature.”
The team, led by Samuel F. Bakhoum from Memorial Sloan Kettering Cancer Center and Ashley M. Laughney from Weill Cornell Medical College, found that chromosomal instability within tumor cells causes prolonged activation of the cGAS-STING pathway, resulting in cell desensitization to inflammatory responses and a rewiring of signaling pathways. This transforms the tumor microenvironment into an immune-suppressive one, facilitating tumor cell metastasis.

Chromosomal instability (CIN) is a phenomenon where cells continually make mistakes in chromosome segregation during mitosis. It is commonly observed in various cancer types and is believed to be associated with tumor metastasis, immune evasion, and therapy resistance.
In their study, Bakhoum and his team first validated in mouse models of triple-negative breast cancer (4T1, EO771.LMB), colorectal adenocarcinoma (CT26), and melanoma (B16F10) that CIN promotes tumor metastasis and activates the cGAS-STING pathway within tumor cells.
By comparing tumor growth and metastasis in mouse models with varying levels of immune function, they found that the ability of tumors with different levels of CIN to metastasize significantly differed in mice with normal immune function, while the difference was smaller in mice with severely compromised immune systems. This suggests that CIN-driven tumor metastasis depends on the immune system.
So, what are the interactions between CIN and the immune system?
Single-cell RNA sequencing revealed that tumors with high CIN levels were enriched with macrophages, dysfunctional T cells, and other immune-suppressive elements, creating an immune-suppressive tumor microenvironment. Conversely, tumors with low CIN levels were enriched with immune-activating cells. Inhibiting the expression of STING protein in tumor cells reduced the impact of CIN on the tumor microenvironment, making it more similar to the low-CIN state.
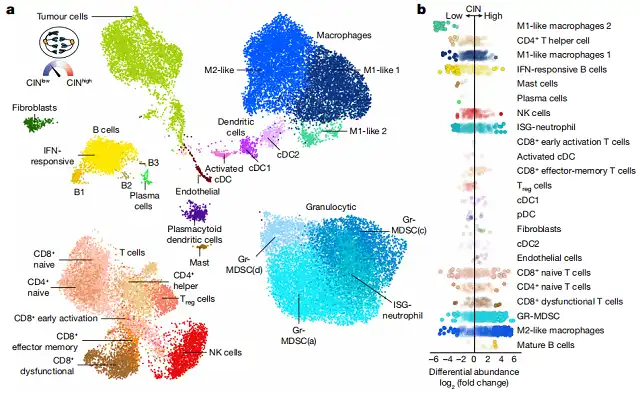
To further understand how CIN reprograms the tumor microenvironment, researchers developed a method called ContactTracing. Based on single-cell RNA sequencing data, ContactTracing analyzes cell characteristics, similar to studying the interests and hobbies of different social groups, to deduce how cells interact with each other and reveal unknown cell-cell interactions.
Using ContactTracing, researchers unveiled the signaling changes within tumor cells of varying CIN levels and how they regulate other cells within the tumor microenvironment. High CIN levels were found to result in prolonged activation of the cGAS-STING pathway within tumor cells, leading to changes in intracellular signaling.
On one hand, endoplasmic reticulum stress signaling pathways were activated, causing tumor cells to secrete immune-suppressive and metastasis-related cytokines, such as CCL2, CXCL1, IL11, affecting immune cells and reshaping the tumor microenvironment. On the other hand, the expression of interferon-stimulated genes (ISGs) was significantly downregulated, preventing tumor cells from producing type I interferon (IFN-1) and responding to its immune response.
In in vitro experiments using human embryonic lung fibroblasts, researchers observed results similar to those in tumor cells when they treated the cells with a STING agonist (cGAMP) daily. The cells quickly became insensitive to the inflammatory response mediated by the STING pathway, with almost no immune response by the fifth day.
Results from in vitro experiments and mouse studies showed that STING inhibitors (C-176, H151) reduced endoplasmic reticulum stress responses and CCL2 secretion in high-CIN tumor cells, significantly extended the survival of tumor-bearing mice, and reduced lung metastatic lesions. Compared to the control group, mice receiving STING inhibitor treatment tolerated it well, and no clinical toxicity was observed. This demonstrates the therapeutic potential of STING inhibitors.

The researchers likened the STING pathway and its mediated inflammatory response to a car alarm. They explained that if it goes off rarely, it grabs your attention. But if it’s constantly blaring, you become accustomed to it and start to ignore it.
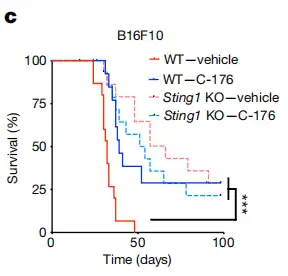
These research findings offer a new treatment strategy for cancer patients with chromosomal instability. In cases where chromosomal instability leads to sustained activation of the STING pathway, some patients’ tumor cells appear to be insensitive to the inflammatory response mediated by STING. In such cases, relying on the power of STING to fight tumors may not be a wise strategy. Instead, these patients may benefit from STING inhibitors.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



