IL-1RAP: A Promising New Target for Cancer Treatment
- Eribulin Shows Superior Survival Benefit for HER2-Negative Metastatic Breast Cancer
- Deadly Drug Made from Human Bones Spreads in West Africa
- Global Cases of Type 1 Diabetes Among the Elderly Triple
- FDA Expected to Expedite Approval Process: Updated COVID-19 Vaccines Could Be Approved This Week
- Pfizer COVID-19/Flu Combo Vaccine Underperforms in Late-Stage Trial
- Understanding Merkel Cell Carcinoma: Causes Symptoms and Latest Treatments
IL-1RAP: A Promising New Target for Cancer Treatment
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
IL-1RAP: A Promising New Target for Cancer Treatment
Cancer remains one of the leading causes of death globally. Despite recent advancements in cancer therapies, such as chimeric antigen receptor T (CAR-T) cells and antibody-drug conjugates (ADCs), there is still a need to identify new targets expressed by tumor cells to develop innovative treatments. Recently, IL-1RAP has shown great potential as a new target for cancer therapy.
IL-1RAP is involved in three signaling pathways of the IL-1 family: IL-1 receptor (IL-1R), IL-33 receptor (IL-33R), and IL-36 receptor (IL-36R). These pathways have demonstrated pro-tumor activity. Moreover, IL-1RAP has been found to be upregulated in several cancers, including hematological and solid tumors (pancreatic, colorectal, and breast cancers).
Currently, two types of therapies targeting IL-1RAP are undergoing clinical trials: CAR-T cell therapy and antibody-mediated cell cytotoxicity (ADCC) or direct blocking IL-1RAP antibody immunotherapy.
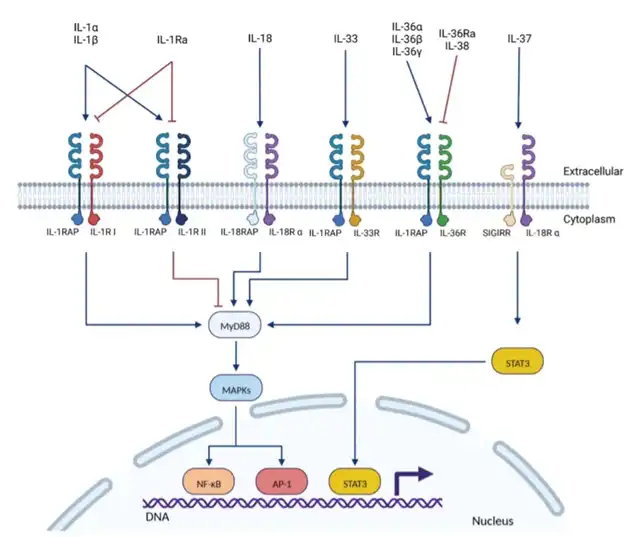
The IL-1 Superfamily
The IL-1 superfamily consists of various cytokines and their receptors. To date, 11 cytokines, 5 receptors, and 5 co-receptors have been described within this family. While most cytokines have been extensively studied, the exact functions of some cytokines (IL-37, IL-38) and receptors (SIGIRR, IL-1RAPL1/2) remain unclear.
Members of the IL-1 family are usually divided into four subfamilies based on their co-receptors: (i) IL-1, (ii) IL-33, (iii) IL-36, which works with the co-receptor IL-1RAP, and (iv) IL-18, which binds to different co-receptors.
Structure and Signaling Pathways of IL-1RAP
IL-1RAP, a member of the IL-1 receptor family, shares a significant molecular homology with other proteins in the IL-1R superfamily. IL-1RAP consists of three immunoglobulin (Ig) extracellular domains (D1, D2, and D3) and a TIR C-terminal intracellular domain. It can activate NF-κB and MAPK pathways.
IL-1RAP can express several isoforms through selective splicing. These include two soluble forms and two membrane forms:
- mIL-1RAP: Initially known as IL1-R3, it participates in synaptic differentiation during immune processes.
- sIL-1RAP: Formed from the extracellular domain of mIL-1RAP, it can bind to the soluble form of IL-1RII and enhance its affinity for IL-1α and IL-1β, playing a role in the negative regulation of IL-1 signaling.
- sIL-1RAPβ: Another soluble form, the increase in which, and the decrease in membrane form, inhibit IL-1 signaling, leading to apoptosis.
- IL-1RAPb (also known as AcPb): Differing from mIL-1RAP by an extended C-terminal domain (an additional 140 amino acids) and a modified TIR domain.
IL-1RAP does not directly bind IL-1. Instead, it forms a complex with IL-1R, enhancing the binding affinity of IL-1 to IL-1R. The general mode of action involves cytokines (IL-1α, IL-1β, IL-33, IL-36α, IL-36β, and IL-36γ) binding to their primary receptors (IL-1RI and IL-1RII, IL-33R/ST2, IL-36R/IL-1Rrp2) and IL-1RAP. This induces the recruitment of IL-1RAP, forming a dimer with the receptor intracellular domain and IL-1RAP’s TIR domain. This dimer recruits and binds several intracellular proteins and kinases such as Tollip, MyD88, IRAK family members, and TRAF-6. These proteins trigger intracellular signaling cascades, inducing NF-κB and AP-1 dependent expression of pro-inflammatory cytokines, chemokines, and inflammatory responses.
Expression of IL-1RAP in Cancer
Over the past few decades, IL-1RAP expression in the tumor microenvironment and on tumor cell surfaces has been extensively studied. Initially observed in hematological cancers like chronic myeloid leukemia (CML) and acute myeloid leukemia (AML), IL-1RAP is also overexpressed in several solid tumors, affecting various organs.
Pancreatic Ductal Adenocarcinoma (PDAC): The most common and aggressive pancreatic cancer type. Immunohistochemistry (IHC) and single-cell RNA-seq studies have shown IL-1RAP overexpression (81%) in PDAC patient samples, with the highest expression in the human A6L cell line.
Ewing Sarcoma: A common bone tumor in children and adolescents characterized by overexpression of CD99. High IL-1RAP mRNA levels and protein expression in multiple Ewing sarcoma cell lines have been confirmed by Western blotting. IL-1RAP overexpression is also observed in non-small cell lung cancer (NSCLC), glioma, triple-negative breast cancer (TNBC), gastric adenocarcinoma, and cervical cancer cell lines.
Role of IL-1RAP in Tumor Progression and Metastasis
Evidence suggests that IL-1RAP plays a crucial role in various stages of tumor promotion, progression, and metastasis. In primary AML cells from patients, IL-1RAP downregulation using shRNA reduced primary cell colony formation and increased apoptosis. Targeting IL-1RAP with blocking antibodies also increased apoptosis and differentiation in THP-1 (AML) cells, reducing cell growth, highlighting IL-1RAP’s role in AML progression.
In PDAC cell line A6L, IL-1RAP inhibition decreased cell viability and colony formation, reduced invasiveness, and caused significant G0/G1 cell cycle arrest. IL-1RAP knockdown cells showed reduced levels of proliferative phosphorylated/activated ERK (MAPK) involved in the IL-1RAP pathway. In gastric adenocarcinoma, IL-1RAP inhibition reduced tumor proliferation, migration, and invasion in vitro and in vivo. These results confirm IL-1RAP’s critical role in both solid and hematological cancers.
Further studies have explored IL-1RAP’s role in immune evasion, tumor progression, and metastasis.
IL-1RAP’s Role in the Tumor Microenvironment (TME)
Inflammation is now recognized as a hallmark of cancer, driven by many interconnected factors in the TME: cytokines, chemokines, innate and adaptive immune cells, fibroblasts, and hypoxia. The IL-1R axis plays a significant role in several immune cells within the TME.
Melanoma: The interaction between tumor cells, macrophages, and fibroblasts in the TME initiates IL-1 signaling cascades, producing CXCR2-stimulated secretion, ultimately increasing melanoma cell resistance to MAPK signaling inhibition therapies like vemurafenib and selumetinib.
Th17 Cells: The IL-1β/IL-1R pathway is crucial for the early differentiation and proliferation of Th17 cells. These cells produce IL-17, promoting tumor growth and angiogenesis in mouse melanoma and bladder cancer.
Oropharyngeal Squamous Cell Carcinoma (OPSCC): In HPV-negative OPSCC, the IL-1/IL-1R axis is responsible for the production of chemokine CXCL8, recruiting neutrophils into the TME. Tumor-associated neutrophils (TAN) are associated with poor prognosis in OPSCC.
Besides directly promoting tumor cell proliferation and growth, IL-1RAP in the TME facilitates angiogenesis, matrix remodeling, and cytokine and growth factor production by TME components. The complex role of the IL-1 superfamily in the TME suggests that different signaling axes can have anti-tumor or pro-tumor functions, depending on cancer type, inflammation status, and interacting cells. Further research in this field may uncover new therapeutic targets or elucidate disease mechanisms.
Targeting IL-1RAP in Cancer Therapy
Currently, therapies targeting IL-1RAP are mostly in preclinical development, with some in clinical stages. Most are based on IL-1RAP blocking antibodies.
Cantargia AB: A Swedish company has been developing IL-1RAP-targeting therapies for various cancers, including CML, AML, and several solid tumors. Their anti-IL-1RAP antibody is in clinical trials, with mid-term safety results indicating good tolerance and no acute toxicity.
CAR-T Cells: A French team has developed CAR-T cells targeting IL-1RAP. Two clinical trials (NCT02842320 and NCT04169022) are ongoing, with results yet to be published.
Conclusion
In the past decade, new research has uncovered the role of IL-1RAP in the IL-1 superfamily signaling pathways and its involvement in tumor development and progression. IL-1RAP is now considered a promising therapeutic target for various hematological and solid cancers. Specific therapies targeting IL-1RAP, including antibody-based and CAR-T cell therapies, have been developed. As experiments progress, this new target holds the potential to bring significant breakthroughs in cancer treatment.
IL-1RAP: A Promising New Target for Cancer Treatment
References
IL-1RAP, a Key Therapeutic Target in Cancer. Int J Mol Sci. 2022 Dec; 23(23): 14918.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.