Mastering Target Antigen Selection for Cancer Antibody-Drug Conjugate
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Mastering Target Antigen Selection for Cancer Antibody-Drug Conjugate
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Mastering Target Antigen Selection for Cancer Antibody-Drug Conjugate
Antibody-Drug Conjugates (ADCs) represent a powerful approach in cancer treatment, combining monoclonal antibodies targeting specific antigens with small molecule cytotoxic drugs linked by a connector.
ADCs leverage the specificity of antibodies for their target antigens, combining the potent cytotoxic effects of small molecule chemotherapy with the tumor-targeting capabilities of antibody drugs.
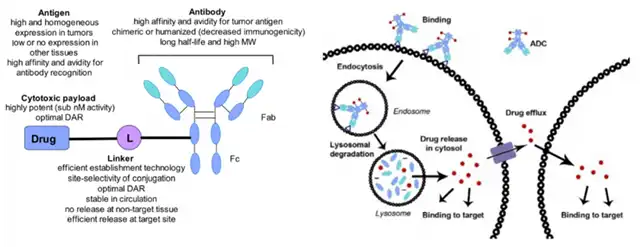
In the development of ADCs, the selection of target antigens that are highly expressed on malignant cells but minimally expressed on normal tissues and immune cells is crucial for achieving selectivity and efficacy while minimizing off-target toxicity.
Additionally, several factors related to the expression patterns and biological characteristics of antigens need to be considered when choosing antigens for ADCs.
Factors in Antigen Selection
Efficient delivery of cytotoxic payloads in ADCs requires a rational selection of target antigens to maximize tumor selectivity and anti-tumor efficacy while minimizing off-target toxicity to normal tissues. Striking a balance between safety and efficacy is paramount in ADC design, emphasizing the importance of delivering payloads to cancer cells based on empirical evidence for each tumor type.
According to established principles, an ideal target antigen for effective ADCs should exhibit sufficient and uniform expression on the surface of tumor cells while having minimal expression on normal cells. This helps limit off-target toxicity to tumor-adjacent healthy tissues, optimizing the therapeutic index. Besides specificity and overexpression, the optimal target antigen should also be extracellular for efficient antibody binding to antigen epitopes.
Furthermore, the effectiveness of ADCs often depends on efficient target-mediated internalization. The rate of internalization and the dynamics of internalization vesicle transport following binding of the tumor antigen to the ADC can directly impact payload release and cancer cell killing.
Understanding the primary direction of antigen targeting, whether towards recycling or lysosomal targeting pathways, is crucial. Antigen-ADC complexes recirculating to the plasma membrane are thought to affect efficient delivery to lysosomes and may hinder payload release to the cytoplasm, potentially compromising ADC efficacy. Another factor affecting ADC effectiveness is the rate of antigen shedding from the cell surface, often mediated by tumor cell-produced proteases in a process known as antigen shedding.
Target Expression Levels
The threshold levels of antigen expression required to achieve ADC activity vary significantly based on several parameters, many of which are not fully elucidated. Known expression levels depend on specific targets, recognized antigen epitopes, and cancer indications, with notable variations observed in ADCs targeting solid tumors. For instance, clinical experience evaluating Kadcyla in HER2-positive metastatic breast cancer suggests that higher expression subgroups exhibit better survival rates compared to low expression subgroups.
However, the remarkable efficacy of Enhertu in HER2-low expression breast cancer patients indicates that there is no universally applicable threshold for target antigen expression to ensure ADC efficacy. In vitro and in vivo experiments show that some HER2-negative cell lines may still maintain active HER2 signaling and sensitivity to anti-HER2 therapy. This suggests that lower levels of pro-tumor signaling may still support tumor growth due to the lower surface expression of HER2. Similar variations exist for other targets; for example, selecting patients with only high FRα expression for ADC targeting seems to be associated with therapeutic benefits. In clinical studies of ADC targeting CD70 in renal cell carcinoma, a limited correlation was observed between antigen expression levels and CD70-targeted ADC sensitivity.
In summary, preclinical research and clinical evaluations of ADCs for various cancer types indicate that there is no overall paradigm associating antigen expression levels with ADC activity. Therefore, the ideal cut-off value for antigen expression needs to be determined empirically for each tumor type and ADC.
Off-Target Toxicity
The most commonly observed toxicity in clinical ADC treatment is due to off-target effects, and the off-target tumor toxicity of ADCs may be influenced by the selection of target antigens.
To mitigate toxicity, consideration must also be given to the physiological role of the target antigen and the mechanisms by which it exerts this role. Therefore, preclinical toxicity studies for new ADC targets not only need to investigate the differential expression of the target between cancer and normal tissues but also need to study the physiological functions of the target to determine potential toxicity. For example, the ADC using the CD44 antibody bivatuzumab with DM-1 for treating squamous cell carcinoma reported fatal skin toxicity, possibly attributed to the expression of CD44 in healthy keratinocytes.
However, despite the expression of the target antigen on normal cells being an important factor to consider, it does not necessarily preclude the development and ultimate success of ADCs. For instance, although TROP-2 is highly expressed in some normal tissues, Trodelvy has been successfully developed and FDA-approved for the treatment of metastatic triple-negative breast cancer. This suggests that the differences in antigen expression between normal and malignant tissues may be sufficient to avoid severe toxicity. Additionally, it is suggested that TROP-2 is expressed intracellularly rather than on the cell surface of normal cells or in locations such as ducts or glandular epithelial lumina inaccessible to antibodies or ADCs, which may play a crucial role.
Antigen Shedding
When selecting ADC targets, the rate of antigen shedding may be an important consideration. In antibody-based therapeutic approaches, antigen shedding refers to the removal of surface-expressed target antigens, a process typically mediated by proteases as a means of functional regulation.
Early studies on immunotoxins suggested that increased antigen shedding might reduce the available quantity of ADCs capable of targeting and binding to tumors, thereby compromising ADC efficacy.
However, other studies using mathematical and experimental models suggest that a high antigen shedding rate may either increase or decrease ADC efficacy, depending on several factors, including ADC internalization rate, ADC recycling rate, and permeation through the tumor microenvironment.
For example, for immunotoxins targeting mesothelin and CD25, the same model suggests that an increase in shedding rate would increase the efficacy of the former but decrease the efficacy of the latter.
ADC Targets for Hematologic Tumors
For hematologic tumors, immune lineage-specific biomarkers such as CD19, CD20, CD22, CD33, BCMA, and CD79 have been widely explored as candidate targets for ADC development. Additionally, approved ADC targets exhibit easy internalization upon binding, a critical characteristic for effective ADCs.
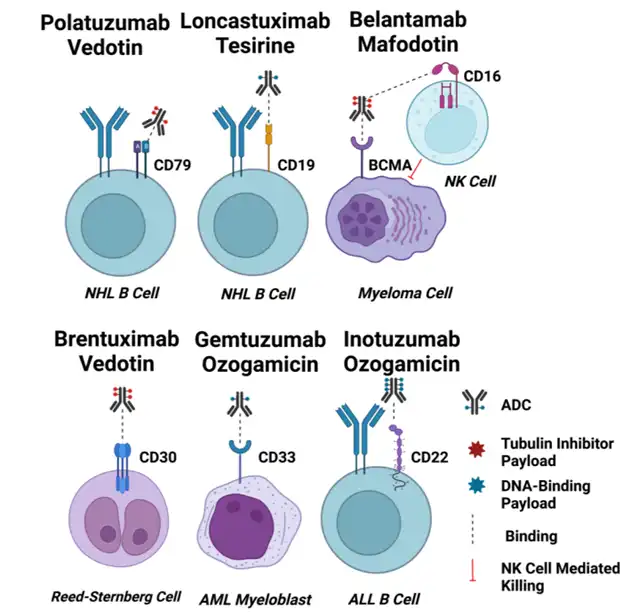
CD33
CD33 is a 67kDa transmembrane sialic acid-binding immunoglobulin-like lectin (SIGLEC) family member receptor. While it is typically expressed on normal myeloid cells, its preferential overexpression on AML cells makes it a target for Gemtuzumab ozogamicin. The internalization of CD33 is regulated by the inhibitory immunoreceptor tyrosine-based inhibition motif (ITIM), and internalization is activated by clathrin-mediated endocytosis (CME). Interestingly, there is no correlation between the expression level of CD33 in AML cells and its internalization rate. CD33 is a slow internalizing antigen, and cross-linking CD33 does not improve internalization. Lack of response to GO in AML patients may be associated with the impaired internalization function of CD33 receptors.
CD22
CD22 is a 140 kDa transmembrane glycoprotein and a member of the SIGLEC family like CD33. The key difference is that CD22 is much larger than CD33, possessing multiple Ig domains and ITIM/ITIM-like motifs. CD22 expression is restricted to B cells, and it is upregulated in B cell malignancies. In normal B cells, CD22 serves as a regulator of B cell activation and a suppressor of B cell receptor signaling. Internalization of CD22 is known to be slow and may involve clathrin-mediated endocytosis. Given its expression pattern and role in B cell signaling, CD22 has been explored as a target for ADCs in B cell malignancies.
CD19
CD19 is a 95 kDa transmembrane glycoprotein expressed on the surface of B cells. CD19 is involved in B cell receptor signaling and is critical for B cell development and survival. It is expressed throughout B cell development, from early pre-B cells to the mature B cell stage. Due to its consistent expression on B cell malignancies, CD19 has been a popular target for various immunotherapies, including CAR-T cell therapies and ADCs.
ADC Targets for Solid Tumors
Selecting suitable antigens for ADC development in solid tumors poses a significant challenge due to the heterogeneity of target expression across patient populations.
Various antigens have been explored as potential targets, but the ideal target should be highly expressed on tumor cells, exhibit limited expression on normal tissues, and enable efficient internalization of the ADC complex.
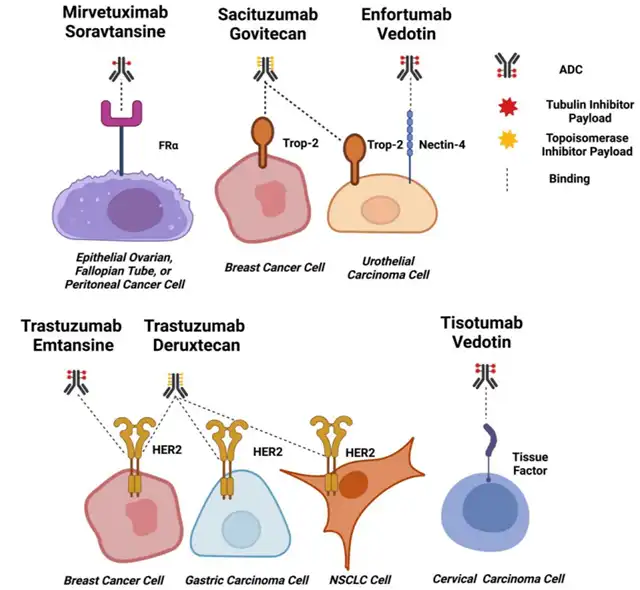
Some common solid tumor ADC targets include:
HER2 (ErbB2)
HER2, also known as ErbB2, is a transmembrane receptor tyrosine kinase that belongs to the epidermal growth factor receptor (EGFR) family. It is overexpressed in a subset of breast, gastric, and other cancers. Trastuzumab emtansine (Kadcyla) is an FDA-approved ADC targeting HER2 for the treatment of HER2-positive breast cancer.
EGFR (Epidermal Growth Factor Receptor)
EGFR is a cell surface receptor that belongs to the ErbB family. It is overexpressed in various solid tumors, including lung, colorectal, and head and neck cancers. Cetuximab is a monoclonal antibody against EGFR, and while not a traditional ADC, it has been used in combination with chemotherapy in the treatment of certain cancers.
TROP-2 (Trophoblast Cell-Surface Antigen 2)
TROP-2 is a cell surface glycoprotein that is overexpressed in various solid tumors, including breast, lung, and pancreatic cancers. Sacituzumab govitecan-hziy (Trodelvy) is an ADC targeting TROP-2 and is approved for the treatment of metastatic triple-negative breast cancer.
Mesothelin
Mesothelin is a glycoprotein that is overexpressed in several solid tumors, including mesothelioma, ovarian, and pancreatic cancers. Anetumab ravtansine is an ADC targeting mesothelin that has been investigated in clinical trials.
PSMA (Prostate-Specific Membrane Antigen)
PSMA is a cell surface protein that is overexpressed in prostate cancer cells. Enfortumab vedotin-ejfv (Padcev) is an ADC targeting Nectin-4 that is approved for the treatment of locally advanced or metastatic urothelial cancer.
Factors Affecting Antigen Selection
-
Expression Levels: The level of expression of the target antigen is a critical factor. Ideally, the target should be highly expressed on tumor cells and minimally expressed on normal tissues.
-
Tumor-Specificity: The target antigen should be specific to tumor cells, and its expression on normal tissues should be limited to reduce off-target toxicity.
-
Internalization Rate: Efficient internalization of the ADC complex is crucial for delivering the cytotoxic payload into cancer cells. The target antigen should be capable of rapid and efficient internalization.
-
Shedding Rate: The rate at which the target antigen is shed from the cell surface can impact the effectiveness of ADCs. High shedding rates may reduce the availability of antigens for ADC binding.
-
Physiological Role: Understanding the physiological role of the target antigen is important to predict potential toxicity. If the target antigen plays a crucial role in normal cell function, its targeting might lead to adverse effects.
-
Antigen Distribution: The distribution of the target antigen on the cell surface and its accessibility to antibodies play a role in ADC effectiveness. In some cases, antigens may be located intracellularly, limiting antibody binding.
-
Tumor Microenvironment: The characteristics of the tumor microenvironment, such as pH and protease levels, can influence the stability and efficacy of ADCs.
-
Heterogeneity: Tumor heterogeneity can impact the selection of target antigens. A target that is uniformly expressed across tumor cells is preferable to minimize the risk of treatment-resistant clones.
-
Immune Response: The immune system’s response to the ADC, including the potential development of anti-drug antibodies, can affect treatment efficacy and safety.
-
Payload Delivery: The payload (cytotoxic drug) should be efficiently delivered to the tumor cells after ADC binding and internalization, leading to cell death.
By considering these factors, researchers aim to identify target antigens that fulfill the criteria of tumor specificity, efficient internalization, and minimal off-target effects, ultimately enhancing the therapeutic window of ADCs.
The complexity of solid tumors, the heterogeneity of antigen expression, and the dynamic nature of the tumor microenvironment contribute to the challenges in ADC development for solid tumor indications.
Conclusion: Mastering Target Antigen Selection for Cancer Antibody-Drug Conjugate
In conclusion, target antigen selection is a critical aspect of ADC design. The choice of the target antigen impacts the specificity, efficacy, and safety of the ADC therapy.
By considering factors such as expression levels, internalization rates, shedding, and off-target toxicity, researchers can optimize the design of ADCs for various cancer types. It’s essential to conduct thorough preclinical studies and understand the biology of the target antigen to predict and mitigate potential toxicities.
Additionally, advancements in our understanding of cancer biology and the tumor microenvironment will likely contribute to the identification of novel and effective target antigens for the development of next-generation ADCs.
Mastering Target Antigen Selection for Cancer Antibody-Drug Conjugate
References:
“Target Antigen Attributes and Their Contributions to Clinically Approved Antibody-Drug Conjugates (ADCs) in Haematopoietic and Solid Cancers.” Cancers (Basel). 2023 Mar; 15(6): 1845.
“Impact of Endocytosis Mechanisms for the Receptors Targeted by the Currently Approved Antibody-Drug Conjugates (ADCs)—A Necessity for Future ADC Research and Development.” Pharmaceuticals (Basel). 2021 Jul; 14(7): 674.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



