11 COVID-19 Vaccines: Comparison and Update
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
11 COVID-19 Vaccines: Comparison and Update
11 COVID-19 Vaccines: Comparison and Update. The first FDA-approved COVID-19 vaccine is here! On December 14, A nurse in New York received the first COVID-19 vaccine in the United States.
On December 11, the U.S. FDA announced the emergency authorization of the new coronavirus vaccine jointly developed by Pfizer and BioNTech in Germany for use in people over 16 years of age.
On December 13, the first batch of delivery trucks for the COVID-19 vaccine in the United States drove out of Pfizer Pharmaceuticals, and the U.S. Federal Marshals escorted the vehicles. The vaccination officially started on December 14.
In addition to the Pfizer/BioNTech COVID-19 vaccine, there is also a vaccine: Moderna will also be approved this month.
At present, COVID-19 vaccines of 11 companies worldwide are in the phase III clinical trials. Among them, the mRNA vaccine BNT162b2, jointly developed by BioNTech/Fosun Pharmaceutical/Pfizer, has been the first to be approved by the FDA for marketing, and the next fastest is Moderna (Moderna) The developed mRNA-1273.
| Company | Vaccines | Types | Trials | Vaccination |
| Pfizer/BioNtech/Fosun | BNT162b2 | mRNA | III | d0, d21 |
| Moderna | mRNA-1273 | mRNA | III | d0.d28 |
| Kexing | PiCoVacc | Inactivated | III | do, d14 |
| SINOPHARM Wuhan | Vero | Inactivated | III | d0, d21 |
| SINOPHARM Beijing | Vero | Inactivated | III | d0, d21 |
| CanSinoBio/Academy of Military Sciences | Ad5-nCoV | Adenovirus vector | III | d0 |
| AstraZeneca/University of Oxford | COV003 | Adenovirus vector | III | d0,d28 |
| Johnson & Johnson | Ad26.COV2.5 | Adenovirus vector | III | d0, d56 |
| Gamaleya | Gam-COVID-Vac | Adenovirus vector | III | d0,d21 |
| Novavax | NVX-CoV2373 | Recombinant protein | III | d9,d21 |
| Bharat Biotech | Covaxin | Inactivated | III | d0,d28 |
01. Pfizer/BioNTech COVID-19 vaccine and Moderna COVID-19 vaccine
Recently, Pfizer/BioNTech’s COVID-19 vaccine and Moderna’s COVID-19 vaccine, these two vaccines have become the focus of attention, let’s talk about these two vaccines first:
Same point
Both COVID-19 vaccines use mRNA technology routes.
At present, the global COVID-19 vaccine mainly adopts five technical routes of nucleic acid vaccine (mRNA vaccine and DNA vaccine), adenovirus vector vaccine, recombinant protein vaccine, and inactivated vaccine.
As a new generation of nucleic acid vaccine research and development technology, mRNA technology has four advantages compared with traditional vaccines:
- Good safety, no virus, no risk of infection
- Fast research and development speed, rapid development, new vaccine candidates;
- Good immunogenicity, dual mechanisms of humoral immunity and T cell immunity, strong immunogenicity;
- High production capacity and easy mass production, supporting the goal of global supply.
Difference:
According to the current clinical trial situation, we mainly compare the following two aspects:
01. The vaccine is effective
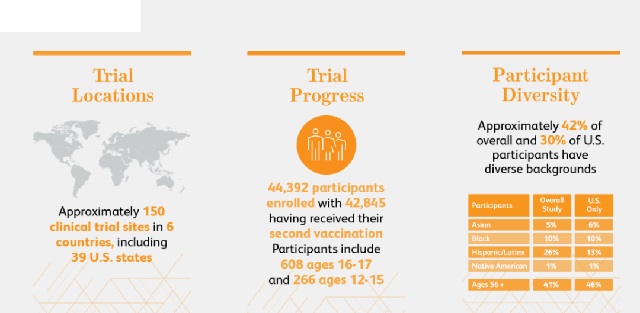
- Pfizer/BioNTech Covid-19 vaccine: Pfizer and BioNTech’s COVID-19 vaccine conducted a final efficacy analysis in a phase 3 clinical trial. The preliminary efficacy analysis showed that the vaccine has an effective rate of 95%.
- Moderna COVID-19 vaccine:Moderna disclosed: The final analysis result of phase III clinical data of the COVID-19 vaccine (mRNA-1273): the preventive efficacy reached 94.1%, slightly lower than the result of the previous interim analysis of 94.5%.
02. Storage and transportation conditions
- Pfizer/BioNTech Covid-19 vaccine:The storage and transportation conditions of Pfizer/BioNTech’s COVID-19 vaccine are relatively harsh. It needs to be transported at minus 70°C and can only be stored for 5 days at 2°C-8°C.However, the CEO of Pfizer said: We are working on a new coronavirus vaccine that does not need to be stored at ultra-cold temperatures, and we will wait and see~
- Moderna COVID-19 vaccine: Moderna’s mRNA-1273 Covid-19 vaccine is stored stably for 30 days at 2°C-8°C.
2. Which countries can get the COVID-19 vaccine?
Since the emergence of the COVID-19 vaccine, it has been of great concern. When will this vaccine be available? According to the latest news, the global vaccinations against the COVID-19 are as follows:
China
In October, the COVID-19 vaccine was launched in some areas of Zhejiang; in November, Sichuan began to vaccinate the COVID-19 vaccine for key populations, and Anhui is expected to launch in the near future;
United Kingdom
On December 8, the United Kingdom launched a large-scale COVID-19 vaccination program. About 50 hospitals provided vaccination services. The first vaccinator was 90-year-old Margaret Keenan;
Japan
Japanese media reported on September 2 that the Japanese government is considering free inoculation of the COVID-19 vaccine for its citizens. The goal is to ensure the number of COVID-19 vaccines by the first half of 2021 and provide all citizens with free COVID-19 vaccination;
United States
On the morning of December 13, U.S. local time, the truck loaded with the first batch of COVID-19 vaccines left the Pfizer factory in Portage, Michigan. The vaccines will be sent to 636 scheduled locations across the United States. The vaccination will officially start on December 14.
Russia
The COVID-19 vaccination electronic application system for citizens of the Moscow City Government of Russia was launched on December 4, and the vaccination station began to work on the 5th;
Malaysia
It was announced on November 27 that the Malaysian government had signed a contract with the multinational pharmaceutical company Pfizer through the Ministry of Health to purchase 12.8 million doses of vaccine from the company to prevent COVID-19 pneumonia.
3. The COVID-19 vaccine is approved for marketing, would you choose to vaccinate?
The COVID-19 vaccine has been successfully developed and approved for marketing. Would you choose to vaccinate?
On this topic, online controversy is still quite big, and netizens have expressed their opinions:
- 01. It was developed in just one year and not enough time, so I dare not fight;
- 02. Don’t fight in a hurry, first look at the reactions of those subjects;
- 03. Wish to get the vaccine now;
In summary, everyone’s views can be divided into two types: one is: someone is on the sidelines, and the other is: I hope early vaccination and early prevention!
For those who dare not fight, it is nothing more than worrying about the safety of the vaccine. Most of them think: The COVID-19 vaccine can be developed so quickly, must it be a shortcut?
What Zhizhi wants to say is: Although it is a shortcut, but within a reasonable range, it does not mean that the vaccine is not safe and effective. Even before subjects are vaccinated, they must undergo strict audits before clinical trials can be conducted.
For example, before subjects are vaccinated with Pfizer/BioNTech’s COVID-19 vaccine, they must be approved by the US Institutional Review Board (IRB).
Here, it is estimated that many people are curious that two foreign COVID-19 vaccines are about to be approved for marketing. Why is China so “low-key”?
The latest news about the Chinese COVID-19 vaccine:
Latest news: Sinopharm’s COVID-19 vaccine was approved for marketing in the UAE on December 9. This is China’s first officially approved COVID-19 vaccine.
It is understood that the results of the International Phase III clinical trial of the COVID-19 inactivated vaccine of Sinopharm Group show that the results of the International Phase III clinical trial of the COVID-19 inactivated vaccine of Sinopharm Group show that after testing 42299 volunteers, the vaccine has The efficiency is 86%, and the seroconversion rate of neutralizing antibody is 99%, which can 100% prevent the conversion of mild COVID-19 disease to moderate and severe disease.
(source:chinanet)
Disclaimer of medicaltrend.org



