Stem cells helped a successful kidney transplantation
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Stem cells helped a successful kidney transplantation
Stem cells helped a successful kidney transplantation. Clinical case: Stem cells helped a successful kidney transplantation, with immune tolerance for up to 18 months!
Stem cells help kidney transplantation, and further solve the troubles of patients with kidney failure! For many patients with advanced organ failure, organ transplantation has become the most effective treatment option. Unfortunately, it is necessary to take long-term immunosuppressive drugs after organ transplantation to avoid graft rejection. These immunosuppressive drugs have great side effects and can greatly increase the risk of infection, malignant tumors, cardiovascular and metabolic complications. .
If the donor-specific immune tolerance is induced, this problem can be overcome and the use of immunosuppressive agents can also be reduced. Recently, according to “Stem Cells Translational Medicine” magazine, a case of a living donor kidney transplant recipient using mesenchymal stem cell infusion to induce immune tolerance successfully. Without the use of immunosuppressive agents, the new organ can be completely tolerated for 18 months.
The world’s first organ transplantation successfully induced immune tolerance
Not long ago, researchers from Italy reported a successful case of a living donor kidney transplant recipient who used autologous bone marrow mesenchymal stem cells (BMSCs) infusion to induce immune tolerance. By the time the researchers published the paper in Stem Cells Translational Medicine, the patient had not received immunosuppressive medication for 18 months.

The researchers said that this case provides new evidence that in living donor kidney transplantation, autologous bone marrow mesenchymal stem cell infusion can safely and completely stop maintenance anti-rejection drugs after transplantation, and finally achieve a state of immune tolerance.
Here’s the thing: In October 2010, a 37-year-old man with end-stage renal disease received a living donor kidney transplant and participated in an in vitro expanded autologous BMSCs pre-transplant infusion. The safety and feasibility Sexual test research. The day before the organ transplantation, the patient underwent autologous BMSCs infusion, and received low-dose rabbit anti-human thymocyte immunoglobulin infusion every day for six days after the organ transplantation.
The patient was transfused with BMSCs before organ transplantation, and his renal function recovered rapidly afterwards, and the function was stable within 2 years after transplantation. At the same time, the blood test results showed that the patient developed tolerance to the donor kidney. The patient’s immunosuppressive agent was gradually reduced until it was completely stopped. As of the time of the report, the patient had not used immunosuppressants for 18 months, and his kidney function was normal after transplantation.
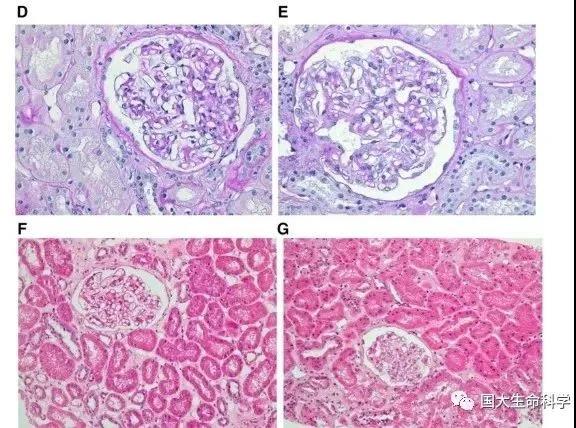
The researchers believe that the success of this case is really attributable to the continuous tolerance-promoting environment induced by stem cell infusion before transplantation.
Outlook
Infusion of stem cells before organ transplantation is one of the latest methods that is expected to overcome immune rejection. Animal experiments have confirmed that the infusion of stem cells before transplantation seems to be an “educational” way to suppress the recipient’s immune rejection of the donor’s organs.
In the future, with the continuous exploration of the mechanism of organ transplant rejection, it is believed that the rejection of transplanted mesenchymal stem cells for allogeneic organ transplantation will eventually mature.
(sourceinternet, reference only)
Disclaimer of medicaltrend.org



