2020 European Neuro-Oncology Society Adult Diffuse Glioma Diagnosis and Treatment Guidelines
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
2020 European Neuro-Oncology Society Adult Diffuse Glioma Diagnosis and Treatment Guidelines
2020 European Neuro-Oncology Society Adult Diffuse Glioma Diagnosis and Treatment Guidelines. Many results have been published on the diagnosis of glioma, and many large-scale clinical trials have obtained mature results. In December 2020, the European Association of Neuro-Oncology (EANO) released the latest guidelines for the diagnosis and treatment of adult diffuse gliomas. This guideline mainly combines the molecular pathology of gliomas (WHO Central Nervous System, 2016). Systematic tumor classification standards and recent results of cIMPACT-NOW) and treatment-related research progress, for reference by medical staff, patients and caregivers.
Editor’s note
- 1. Bevacizumab is approved for the treatment of recurrent glioblastoma in many countries, but it has no significant effect on the prolongation of overall survival.
- 2. Tumor electric field therapy (TTF) combined with temozolomide adjuvant chemotherapy can also prolong PFS and OS in patients, but its feasibility and cost are still controversial.
Original link: https://www.nature.com/articles/s41571-020-00447-z
A summary of the updated guidelines is as follows:
Medical history and clinical examination
Recommend:
Clinical decision-making should take into account KPS score, neurocognitive function, age, and individual risks and benefits. (Evidence level: IV, recommendation level: A) (changes from the 2017 version)
Screening and prevention have no obvious significance for patients with glioma. (Level of Evidence: IV, Level of Recommendation: C)
Patients with genetic mutations or suspected tumor syndromes should receive genetic counseling, and molecular genetic testing may be required thereafter. (Level of Evidence: IV, Level of Recommendation: C)
Preoperative diagnosis
recommend:
The preferred method of imaging diagnosis is MRI. (Level of Evidence: IV, Level of Recommendation: B)
Within a few months after local interventional therapy (including radiotherapy and experimental local therapy), patients with neuroimaging abnormalities need to be considered for false progress. (Evidence level: IV, recommendation level: B) (changes from the 2017 version)
Organizational acquisition
recommend:
Only in very special circumstances can clinical decision-making be considered when the histopathological diagnosis is missing. (Level of Evidence: IV, Level of Recommendation: N/A)
Integrated tissue molecular typing
The diagnosis process should follow the WHO 2016 classification and follow-up recommendations from cIMPACT-NOW (Figure 1).
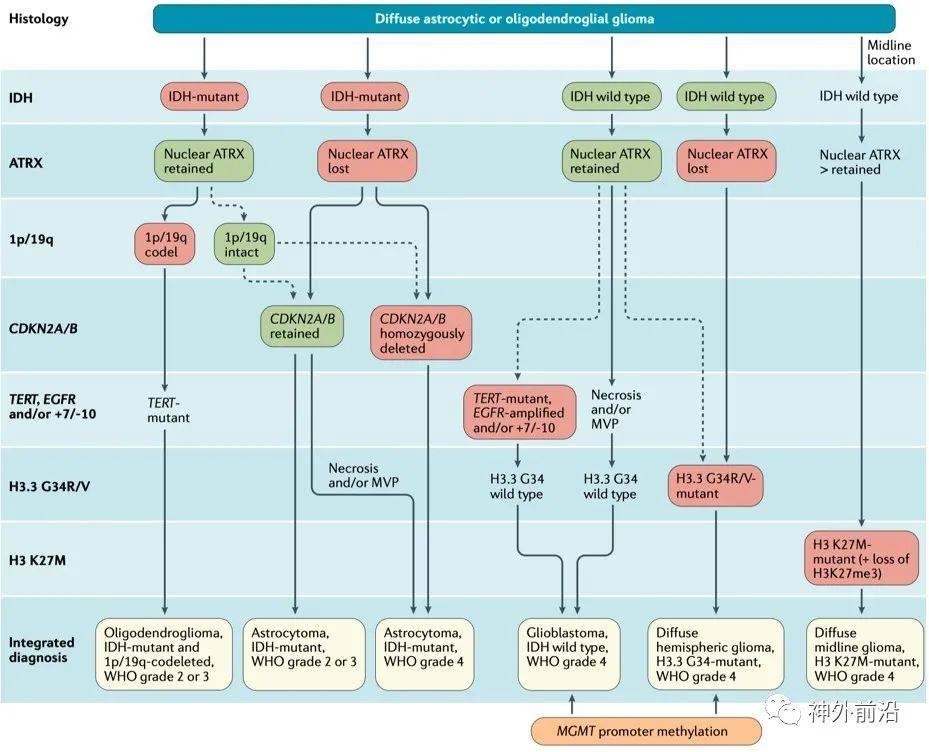
Figure 1 Comprehensive classification of adult diffuse glioma
The following molecular markers are the key to tumor classification: IDH mutation, chromosome 1p/19q co-deletion, histone H3 K27M mutation, histone H3.3 G34R/V mutation, TERT promoter mutation, EGFR amplification, chromosome 7 acquisition and Chromosome 10 deletion (+7/-10) and CDKN2A/B homozygous deletion. IDH mutant astrocytomas can be divided into astrocytoma, IDH mutation, WHO grade 2, astrocytoma, IDH mutation, WHO grade 3 (replaced “anaplastic astrocytoma, IDH mutation, WHO grade 3 “) and astrocytoma, IDH mutation, WHO grade 4 (replaced “glioblastoma, IDH mutation, WHO grade 4”). The term “glioblastoma” is no longer used to refer to IDH mutant astrocytomas. CDKN2A/B homozygous deletion is also a hallmark of WHO grade 4 IDH mutant astrocytoma, suggesting the poor prognosis of IDH mutant diffuse astrocytoma and oligodendroglioma.
Astrocytic gliomas with wild-type IDH and histone H3 status accompanied by necrosis and/or microangiogenesis are classified as IDH wild-type WHO grade 4 glioblastoma. When there is no necrosis or microvascular proliferation, genetic changes such as EGFR amplification, TERT promoter mutation and/or chromosome +7/-10 should be evaluated. If more than one of these changes is present, the tumor should be classified as IDH wild-type glial Blastoma.
H3 K27M mutant WHO grade 4 diffuse midline glioma is defined as a diffuse midline structure, and the 27th amino acid of histone H3.3 or histone H3.1 is mutated from lysine to methionine Glioma. H3 K27M mutant diffuse midline gliomas are typically characterized by positive nuclear immunostaining for H3 K27M and the corresponding loss of nuclear staining for K27 trimethylated histone H3 (H3K27me3). H3.3 G34 mutant WHO grade 4 diffuse hemispheric glioma is considered to be a new subtype of malignant glioma, characterized by a missense mutation in codon 34 of H3F3A.
recommend:
The classification of gliomas should follow the latest WHO classification of central nervous system tumors, supplemented by cIMPACT-NOW updates. (Evidence level: IV, recommendation level: B) (changes from the 2017 version)
In the diagnostic evaluation, immunohistochemical detection of IDH1 R132H mutation and ATRX expression should be routinely performed. (Level of Evidence: IV, Level of Recommendation: B)
If the immunohistochemical result of IDH1 R132H is negative, IDH1 132 code should be tested in all patients with WHO grade 2 and 3 diffuse astrocytoma and oligodendroglioma and glioblastoma patients younger than 55 years old. And IDH2 172 codons were sequenced for diagnosis and treatment according to WHO classification. (Level of Evidence: IV, Level of Recommendation: B)
The 1p/19q co-deletion status of gliomas expressing ATRX and IDH mutations should be determined. (Level of Evidence: II, Level of Recommendation: B)
The methylation status of the MGMT promoter in glioblastoma should be determined, and the use of temozolomide should be assisted. (Level of Evidence: I, Level of Recommendation: B)
CDKN2A/B homozygous deletion should be detected in IDH mutant astrocytoma. (Evidence level: IV, recommendation level: B) (changes from the 2017 version)
For IDH wild-type diffuse gliomas without microvascular proliferation and necrosis, detection of chromosome 7 acquisition and chromosome 10 deletion (+7/-10), EGFR amplification and TERT promoter mutations should be tested to diagnose IDH wild-type glial Blastoma. (Evidence level: IV, recommendation level: B) (changes from the 2017 version)
H3 K27M status should be assessed in diffuse gliomas involving the midline. (Level of Evidence: IV, Level of Recommendation: B)
The BRAF V600 mutation of IDH wild-type diffuse glioma can be evaluated. (Level of Evidence: IV, Level of Recommendation: C)
Treatment plan (uniform recommendation)
Surgical treatment
recommend:
The scope of surgical resection is related to the patient’s prognosis, and it is reasonable to try to perform total tumor resection. (Level of Evidence: IV, Level of Recommendation: B)
At present, the prevention of new permanent neurological deficits after surgery is more important than the scope of resection. (Evidence level: IV, recommendation level: C) (changes from the 2017 version)
Radiation Therapy
It is recommended to start radiotherapy 3-5 weeks after surgery, with a usual dose of 50-60Gy, 1.8-2Gy each time. Hypofractionated radiotherapy can be used for patients older than 65-70 years or with a KPS score less than 70.
The traditional radiotherapy target area usually extends 1.0~2.0cm on the basis of the tumor to cover the microscopically infiltrated tissue, and is adjusted according to the imaging results and anatomical structure, while increasing the 0.3~0.5cm to reduce the influence of the patient’s position. More advanced precision radiotherapy technology can increase the coverage of tumors and reduce the impact on non-tumor tissues. MRI records 3-4 weeks after the end of treatment can be used as a baseline for judging progress and treatment effects.
Medical treatement
Temozolomide is the most commonly used drug for the treatment of glioma, with relatively good safety. Common adverse reactions include thrombocytopenia and liver function involvement. Nitrosourea may cause leukopenia and thrombocytopenia with a certain degree of delay. Patients with more serious adverse reactions need to reduce the dose or terminate the treatment. When using alkylating agents, it is necessary to comprehensively consider its efficacy, long-term toxicity and the possibility of inducing hypermutation, which is especially important for patients with IDH mutations with better prognosis. Bevacizumab is approved for the treatment of recurrent glioblastoma in many countries, but it has no significant effect on the prolongation of overall survival.
Treatment monitoring and follow-up evaluation
Imaging examinations should be performed 2-6 months after the completion of the treatment, and longer intervals may be considered for patients with relatively stable conditions. If recurrence is suspected, check again within 4-8 weeks. False progress or false reaction may occur within 3 months or longer after the start of treatment. If it cannot be confirmed, it should be checked again 4-8 weeks later.
Doctors should recommend psychological counseling and other palliative treatments that can improve the quality of life for glioma patients.
Treatment plan (special recommendation)
IDH mutation and 1p/19q co-deleted oligodendroglioma, WHO grade 2:
Surgical resection is preferred. For patients who have undergone complete resection or incomplete resection but are younger than 40 years old and have no symptoms/only epilepsy, follow-up can be observed. The standard postoperative treatment is radiotherapy sequential PCV treatment. The choice of treatment plan for relapsed patients depends on the initial plan.
IDH mutation and 1p/19q co-deleted oligodendroglioma, WHO grade 3:
For patients who are completely resected, younger than 40 years old, without neurological dysfunction, especially without homozygous CDKN2A/B loss, follow-up can be observed after surgery. The PCV regimen is the current standard first-line treatment. The treatment of advanced patients depends on the choice and response of first-line treatment, and second surgery may be considered. If the first-line treatment is ineffective or tolerable, bevacizumab can be used to control symptoms, but its effect is uncertain.
IDH mutant astrocytoma, WHO grade 2:
Surgical resection of the largest range is preferred. For complete resection, patients younger than 40-45 years with no symptoms or only epilepsy can be observed and followed up; for patients with incomplete resection and/or over 40 years old, local radiotherapy should be considered. The standard postoperative treatment of patients is PCV treatment after radiotherapy. The treatment plan for progressive patients depends on the neurological status, progression pattern and the effect of first-line treatment. Can consider a second operation, postoperative temozolomide adjuvant treatment. Patients who have not received radiotherapy can be supplemented with postoperative radiotherapy.
IDH mutant astrocytoma, WHO grade 3:
The standard treatment is radiotherapy after surgical resection or biopsy. 12 courses of adjuvant temozolomide treatment after radiotherapy can significantly prolong OS, and can also be considered as standard treatment. The treatment of relapsed patients depends on the first-line treatment plan (Figure 2). All patients can consider a second operation, and patients can receive radiotherapy again at least 12 months after the first radiotherapy. Patients who relapse after radiotherapy and have no history of chemotherapy can choose alkylating chemotherapy. The addition of bevacizumab to temozolomide for recurrent gliomas with IDH mutations and no 1p/19q co-deletion does not prolong PFS or OS.
recommend:
For IDH-mutated astrocytoma, standard treatment for WHO 2 patients includes surgical resection or biopsy, followed by radiotherapy and adjuvant PCV chemotherapy. (Level of Evidence: II, Level of Recommendation: B)
IDH mutant astrocytoma, standard treatment for patients with WHO level 3 includes surgical resection or biopsy, followed by radiotherapy and adjuvant temozolomide chemotherapy. (Level of Evidence: II, Level of Recommendation: B)
Recommendation: For oligodendroglioma with IDH mutation and 1p/19q co-deletion, further treatment of patients with WHO grade 2 should be radiotherapy followed by PCV chemotherapy. (Level of Evidence: III, Level of Recommendation: B)
For oligodendroglioma with IDH mutation and 1p/19q co-deletion, patients with WHO grade 3 should receive radiotherapy first and then PCV chemotherapy. (Level of Evidence: II, Level of Recommendation: B)
For most patients with grade 2/3 gliomas with IDH mutations, temozolomide chemotherapy is an effective treatment for the progression after surgical resection and radiotherapy. (Level of Evidence: II, Level of Recommendation: B)
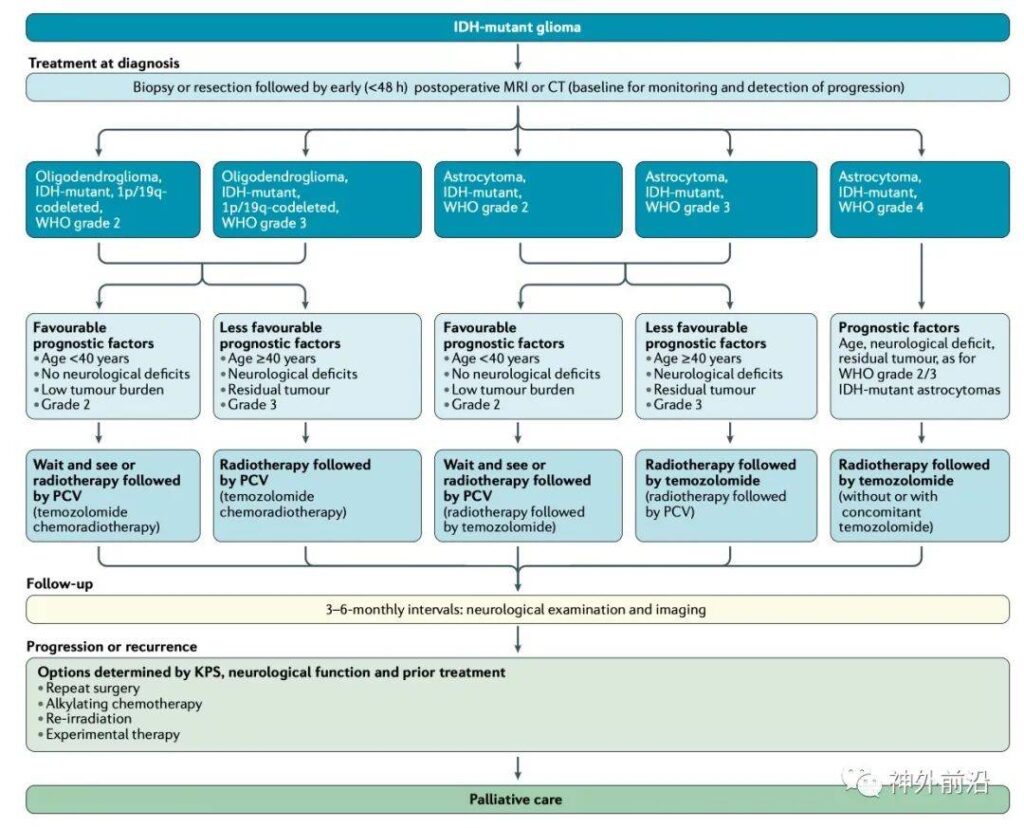
Figure 2 Clinical diagnosis and treatment path of IDH mutant glioma
IDH wild-type glioblastoma, WHO grade 4:
Glioblastoma surgery should be removed as completely as possible. Radiotherapy has always been the standard treatment for glioblastoma. Patients with unfavorable prognostic factors can be treated with hyperfractionated radiotherapy. Temozolomide simultaneous radiotherapy and chemotherapy plus 6 cycles of adjuvant chemotherapy is the standard treatment for newly diagnosed glioblastoma patients with general conditions and good nervous system conditions and less than 70 years old. It is mainly beneficial to the methylated glioblastoma cells of the MGMT promoter Tumor patients. Tumor electric field therapy (TTF) combined with temozolomide adjuvant chemotherapy can also prolong PFS and OS in patients, but its feasibility and cost are still controversial.
The standard treatment for recurrent glioblastoma has not been clearly defined. The choice of regimen is based on previous treatment, age, KPS, MGMT promoter methylation status and disease progression pattern (Figure 3). The main systemic treatment options include nitrosourea, temozolomide reuse, and bevacizumab. At present, there is no drug that can be used in combination with bevacizumab to effectively prolong OS. Its main value is symptom control and reduction of glucocorticoid use.
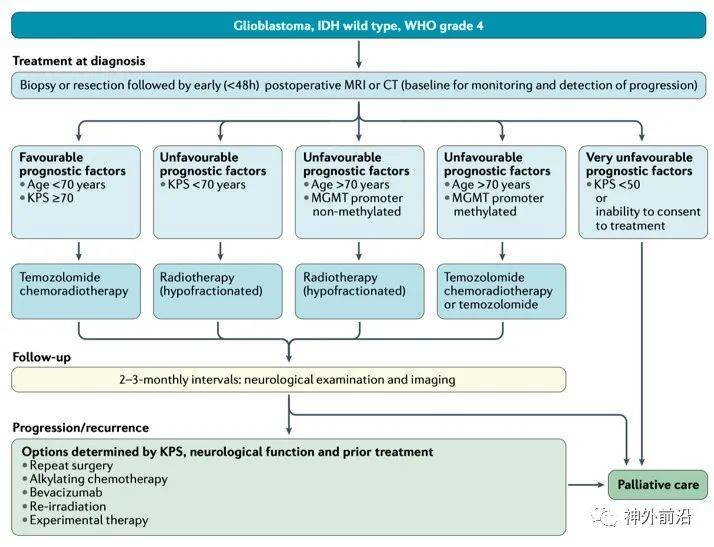
Figure 3 IDH wild-type glioblastoma, WHO level 4 clinical diagnosis and treatment path
recommend:
Standard treatment for glioblastoma patients with IDH wild-type, age less than 70 years old, and KPS greater than 70 include surgical resection or biopsy, followed by concurrent chemotherapy and 6 cycles of adjuvant temozolomide chemotherapy. (Level of Evidence: I, Level of Recommendation: A)
Temozolomide may only be effective in patients with methylation of the MGMT promoter, but not effective in patients with unmethylated. (Level of Evidence: II, Level of Recommendation: B)
Elderly patients who do not consider concurrent radiotherapy or adjuvant radiochemotherapy for temozolomide should receive radiotherapy or temozolomide alone according to the methylation status of the MGMT promoter. (Level of Evidence: II, Level of Recommendation: B)
The standard diagnosis and treatment after recurrence is not clearly defined. Surgery and radiotherapy can be considered, nitrosourea regimen, temozolomide re-use and bevacizumab can be considered, but the significance of bevacizumab for OS has not been confirmed. Consider participating in clinical trials. (Level of Evidence: II, Level of Recommendation: B)
Sum up
With the improvement of the classification of central nervous system tumors, the routine diagnosis and treatment process of glioma has also changed. The diagnosis and treatment plan should follow the recommendations of multidisciplinary consultation, and consider neurological rehabilitation training and treatment. New developments in the guidelines will be updated on the EANO website, and more high-quality RCT studies are needed to supplement the treatment recommendations in the existing guidelines.
2020 European Neuro-Oncology Society Adult Diffuse Glioma Diagnosis and Treatment Guidelines
(sourcesohu, reference only)
Disclaimer of medicaltrend.org



