FDA approved the first anti-VEGFR/EGFR-TKI combination program for first-line NSCLC
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
FDA approved the first anti-VEGFR/EGFR-TKI combination program for first-line NSCLC
FDA approved the first anti-VEGFR/EGFR-TKI combination program for first-line NSCLC. Worldwide lung cancer is the leading cause of cancer deaths, with nearly 1.8 million deaths worldwide each year.
“At present, it has been clinically recognized that the disordered growth of tumors is not only attributed to its malignant potential for continuous proliferation, but also that the host provides oxygen and nutrients to the tumor through angiogenesis and other means is also a key factor in “fueling the flames.” Therefore, at present, inhibiting tumor growth Angiogenesis has been recognized as a relatively effective anti-cancer strategy. Tumor angiogenesis is a key step in the development and metastasis of solid tumors, and it is also one of the main research areas of tumor targeted therapy.
Globally, lung cancer is the leading cause of cancer deaths, with nearly 1.8 million deaths worldwide each year. Stage IV NSCLC is a very refractory cancer with a poor prognosis. 50% of NSCLC patients present with advanced or metastatic disease at the time of diagnosis, and the five-year survival rate for metastatic NSCLC is 6%. 1,2
On May 29, 2020, the U.S. Food and Drug Administration (FDA) approved the combination of VEGFR2 antagonist ramucirumab (CYRAMZA, ramucirumab) and EGFR-TKI erlotinib (Tavceva, erlotinib) for EGFR No. 19 First-line treatment of metastatic non-small cell lung cancer with deletion mutation (19del) and L858R mutation in exon 21. 3
At the same time, this is the first and only anti-VEGFR/EGFR-TKI approved by FD A for the treatment of patients with EGFR-mutant mNSCLC, which can inhibit the VEGFR and EGFR pathways together, which is an important milestone. Compared with erlotinib, the median progression-free survival of the ramucirumab combination program was extended by 7 months to 19.4 months, while reducing the risk of disease progression or death by 41%.
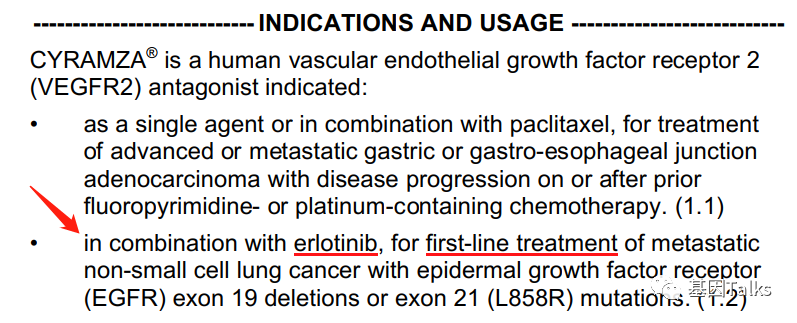
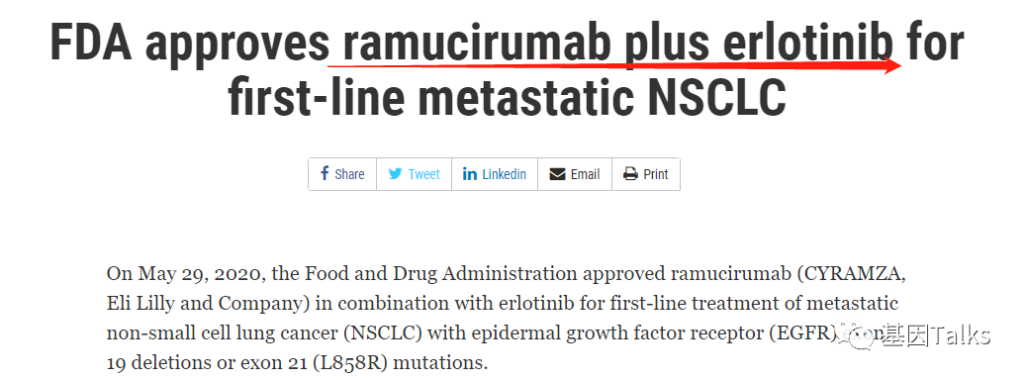
▲ FDA approves ramucirumab combination regimen for mNSCLC patients 3,4
About EGFR
EGFR is a protein that helps cells grow and divide.
Mutations in the EGFR gene may cause the protein to become overactive, leading to faster cell growth and division. EGFR mutations in Asian lung adenocarcinoma patients are as high as 40% to 60%, regardless of race, these mutations are common in women, non-smokers and patients with adenocarcinoma histology.
▲ The function of EGFR protein
In NSCLC tumors with EGFR mutations, 19dels and L858R are the most common activating mutations, accounting for about 90%. These EGFR activating mutations are related to the sensitivity of EGFR-TKIs small molecule targeted drugs (erlotinib, etc.).
About VEGFR
In tumor patients, angiogenesis creates new blood vessels, which provide the tumor with its own blood supply (oxygen, nutrients, etc.) to allow it to grow and spread.
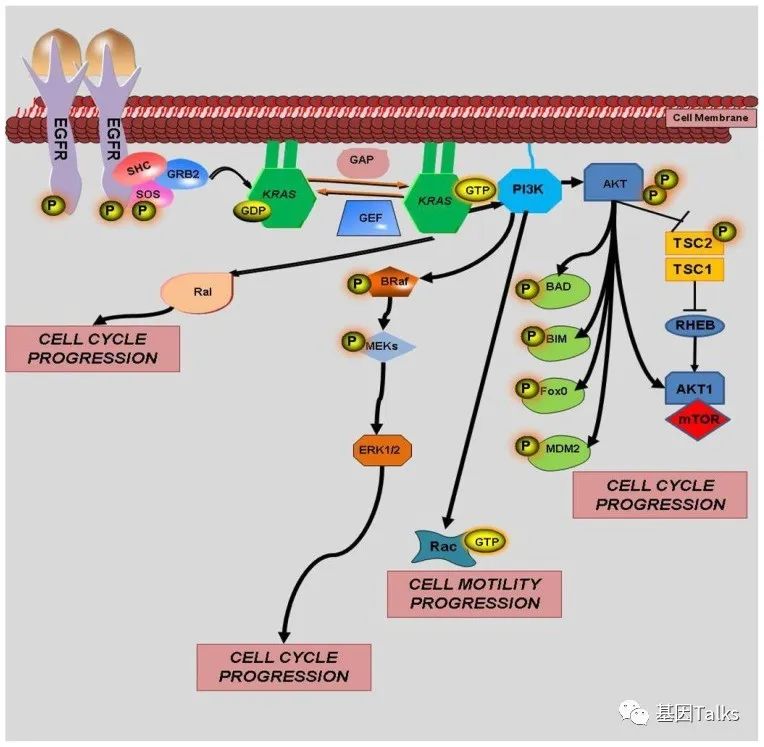
Some tumors can produce a protein called VEGF (vascular endothelial growth factor), which attaches to the VEGF receptor (VEGFR) of vascular cells to form new blood vessels around the tumor and promote its growth. Among the three known VEGFRs (VEGFR1/2/3), VEGFR2 has the closest relationship with VEGF-induced tumor angiogenesis.
After the combination of VEGFR2 and VEGF, it dimerizes and the intracellular tyrosine residues themselves are phosphorylated. Its activity can promote the proliferation, migration and survival of endothelial cells. It should be specially pointed out that the downstream of VEGFR2/VEGF binding Signal effects include: integrin activation through the PI3K/Akt pathway, and induction of endothelial cell growth through activation of the Raf/MEK/Erk pathway.

▲ The role of VEGF and VEGFR
Preventing VEGF protein from binding to receptors located on the surface of blood vessels can inhibit tumor growth by slowing down angiogenesis and nourishing the blood supply of tumors. Because tumors need nutrients delivered by blood vessels to grow and spread, the goal of anti-angiogenesis therapy is to “starve” the tumor.
Ramucirumab is an anti-angiogenic therapy. It is a vascular endothelial growth factor receptor 2 (VEGFR2) antagonist that specifically binds to VEGFR2, thereby blocking the binding of receptor ligands (VEGF-A, VEGF-C and VEGF-D), which may slow down Tumor growth.
The combination of anti-VEGFR/EGFR-TKI in the treatment of EGFR-mutant mNSCLC patients can inhibit the VEGFR and EGFR pathways together. It is an important milestone and will also provide a new first-line treatment option for patients with metastatic EGFR-mutant NSCLC.
About the RELAY test
The FDA’s approval is based on the FDA’s Oncologic Drugs Advisory Committee (FDA’s Oncologic Drugs Advisory Committee) on the basis of a 6-to-five vote of weak recommendations, and the vote is based on the results of the RELAY Phase III trial.
The detailed efficacy and safety results of RELAY were published in the journal “The Lancet Tumor”5. The results showed that in the first-line treatment of EGFR-mutated NSCLC patients, compared with erlotinib alone, in erlotinib Adding ramucirumab can reduce the risk of disease progression or death by 41%. During a median follow-up of 20.7 months, the median progression-free survival (PFS) of the ramoxiimab regimen evaluated by the researchers and erlotinib alone were 19.4 months and 12.4 months (HR=0.59) ), the median progression-free survival was extended by 7 months.

▲ RELAY efficacy and safety results published in “Lancet Oncol”
The RELAY trial (NCT02411448) is a multinational, randomized, double-blind, placebo-controlled, multi-center clinical trial. It recruited 449 cases of EGFR exon 19 deletion mutations (19del) and 21 Patients with metastatic NSCLC with exon L858R mutation.
The recruited patients were randomly assigned 1:1. The experimental group (N=224) received the combination therapy of ramucirumab 10 mg/kg intravenously once every two weeks + erlotinib 150 mg orally once a day, and the control group (N =225) Receive a placebo intravenous infusion every two weeks + 150 mg of erlotinib orally once a day until the disease progresses or unacceptable toxicity occurs.
The main efficacy indicator is progression-free survival (PFS) (RECIST 1.1) assessed by the investigator. Other efficacy outcome indicators include overall survival (OS), overall response rate (ORR) and duration of response (DoR). The median PFS in the ramucirumab plus erlotinib group was 19.4 months, while that in the placebo plus erlotinib group was 12.4 months (HR=0.59, p <0.0001).
The ORR of ramucirumab plus erlotinib group was 76%, the ORR of placebo combined erlotinib group was 75%, and the median DoR was 18.0 months and 11.1 months, respectively. At the time of the final analysis of PFS, OS data were not yet mature, because only 26% of the deaths required for the final analysis had occurred (HR=0.83). 3,4,5

▲ Efficacy data of RELAY trial
Ramucirumab combined with erlotinib has a higher incidence of adverse reactions than placebo combined with erlotinib (≥22% vs ≥2%). It is the most common adverse reaction, including infection, hypertension, and Stomatitis, proteinuria, hair loss, nose bleeding and peripheral edema. The most common laboratory abnormal results (≥22% vs ≥2%) are elevated alanine aminotransferase, elevated aspartate aminotransferase, anemia, thrombocytopenia, neutropenia, and elevated alkaline phosphatase levels Hyperkalemia and hypokalemia.
The FDA Oncology Drug Advisory Committee voted 6 to 5, with 1 vote “nearly win”. Of course, FDA approval may or may not refer to the advice of the Advisory Committee. 6 Why is ramucirumab + erlotinib “controversial”?
1. The current first-line standard for EGFR-mutant mNSCLC is osimertinib, ramucirumab+erlotinib and osimertinib mPFS are not much different (18.9 months vs. 19.4 months), and the clinical use of erlotinib Therefore, some experts believe that the advantages may not be obvious;
2. The effect of brain metastasis treatment is unknown; 3. The adverse reactions increase. Of course, the FDA approved the ramucirumumab + erlotinib combination therapy program for first-line EGFR-mutant mNSCLC patients, indicating that the ramucirumumab + erlotinib combination therapy program is beneficial to the first-line treatment of these patients Benefit/risk ratio. New treatment options can help oncologists have more choices in how to use existing drugs when discussing decisions with patients.
FDA approved the first anti-VEGFR/EGFR-TKI combination program for first-line NSCLC
FDA approved the first anti-VEGFR/EGFR-TKI combination program for first-line NSCLC
(source:internet, reference only)
Disclaimer of medicaltrend.org



