China COVID-19 DNA vaccine with 5-year shelf life
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
China COVID-19 DNA vaccine with 5-year shelf life
China COVID-19 DNA vaccine with 5-year shelf life. Since the outbreak of the COVID-19 epidemic, the development of vaccines has been performing “speed of life and death.” At present, preventive vaccines are being developed rapidly and are now being vaccinated on a large scale worldwide. In this case, the “stability” that determines the convenience of transportation and storage has become a hard indicator for testing a vaccine.
In China’s new coronavirus vaccine team, there is a DNA vaccine that has a shelf life of up to 5 years at standard refrigeration temperatures (2-8°C), and can remain stable for more than 1 year at room temperature. Once it is put into use, it will bring huge benefits to the popularization of vaccination. convenient.

This DNA vaccine was jointly developed by the visitor officer of Village B, Ai Di Weixin, and the American biopharmaceutical company INOVIO, and is currently conducting phase 2 clinical trials simultaneously in China and the United States. Due to the high technical threshold, no DNA vaccine has been successfully marketed in the world. This is also China’s first preventive DAN vaccine approved to enter the clinic.
Ai Diweixin’s strategic director, Dr. Xiaoyan Liu, said in an interview with The Paper recently that “this vaccine may become the first DNA vaccine approved for marketing in history, and it will mark China’s leading level in the field of nucleic acid vaccines in the world.”
DNA vaccines and mRNA vaccines are both nucleic acid vaccines, which are called “third-generation vaccine technology” in the industry. At the end of 2020, the COVID-19 mRNA vaccine has achieved a breakthrough from zero to one, and the development of DNA vaccines has also been closely watched by the industry.
What is the COVID-19 DNA vaccine?
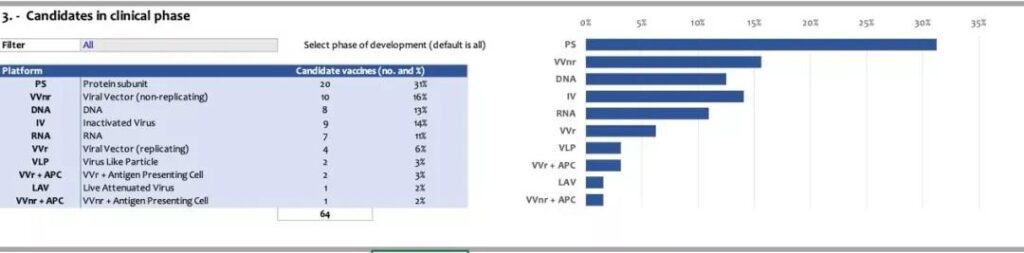
In the global competition of COVID-19 vaccines, China’s three inactivated vaccines have always led the way, becoming one of the most familiar technical routes for the public. In fact, the COVID-19 DNA vaccine also has many scientific research teams “betting”. According to the latest data released by WHO on January 22, 2021, Central European Time, there are currently 64 COVID-19 vaccine candidates in the world that have entered the clinical research phase. Among them, 9 inactivated vaccines (accounting for 14%) and DNA vaccines 8 models (accounting for 13%), in terms of quantity, the two are evenly matched.
WHO official website map
The COVID-19 DNA vaccine is also one of the five technical routes deployed after the epidemic in China, which belongs to the nucleic acid vaccine technical route. At that time, Ai Diweixin’s COVID-19 DNA vaccine project was approved by the Ministry of Science and Technology, and was selected by the State Council for the joint prevention and control of new coronavirus pneumonia vaccine tasks, and received continuous follow-up and support from the Ministry of Science and Technology and the National Health Commission.
Liu Xiaoyan introduced that the principle of DNA vaccine is to make the DNA encoding the antigen protein into a vaccine, enter the human body and express the antigen in the body to induce the human body to produce an immune response, so that the vaccinators can obtain the corresponding immune protection ability and achieve the purpose of disease prevention.
After the new coronavirus DNA vaccine is immunized, it will help the human body to produce memory T and B cells. When it encounters the new coronavirus invasion again, it can quickly produce a large number of effector T cells and B cells, neutralize the invading virus through antibodies, and pass T cells ( Cellular Immunity) to eliminate viruses that have infected cells.
Li Bin, deputy director of scientific research at the Shanghai Institute of Immunology, Shanghai Jiao Tong University School of Medicine, introduced in an interview with The Paper that the inactivated vaccine, as a traditional classic technical route, uses an in vitro culture method to prepare antigens, and then inject viral antigen proteins into the human body. Activate the human immune response; and the DNA vaccine of the new generation of technology routes directly input genetic coding information into the human body to produce antigen proteins in the body and activate the immune response.
Li Bin said that the biggest advantage of DNA vaccines is that they can be stored for a long time at room temperature.
INOVIO issued a statement on its official website on December 24, 2020 local time, stating that INO-4800 (the COVID-19 DNA vaccine developed by INOVIO and Ai Diweixin) can remain stable for more than 1 year at room temperature, at 37°C. It can be kept stable for more than one month. Its design shelf life is 5 years at standard refrigeration temperature (2-8°C), and it does not need to be frozen during transportation and storage.
“These are the decisive factors in the fight against the COVID-19 and the timely distribution of vaccines around the world.” The statement said.
Liu Xiaoyan introduced that the “5-year shelf life” is based on the results of long-term stability studies at room temperature for other DNA vaccine products developed by INOVIO. According to INOVIO’s official website, the company has extensive experience in coronaviruses and has developed a DNA vaccine for Middle East Respiratory Syndrome (MERS) caused by related coronaviruses. The vaccine is INO-4700.
“DNA vaccines are very stable and can be stored for a long time at room temperature. They are suitable for remote areas or as a strategic reserve for vaccines.” Liu Xiaoyan said. Both are nucleic acid vaccines, and the transport of mRNA vaccines is inseparable from the cold chain. In terms of the two COVID-19 vaccines currently in use worldwide, Pfizer/BioNTech’s vaccine needs to be transported at -80°C to -70°C, while Moderna’s vaccine needs to be transported at -20°C .
Liu Xiaoyan said that the advantages of DNA vaccines also reflect production. He introduced that the DNA vaccine is produced by E. coli fermentation, the expression system is very mature, can be quickly mass-produced, the process is mature, and the cost is low.
INO-4800 is conducting clinical trials simultaneously in China and the United States
In July 2020, Ai Diweixin’s COVID-19 DNA vaccine was approved to enter clinical trials in China. The Phase 1 clinical trial has been successfully carried out in Shanghai Huashan Hospital. In September of the same year, the first subject was vaccinated. All 45 healthy subjects have now been vaccinated with two injections and entered the follow-up phase. In December 2020, this DNA vaccine officially entered a phase 2 clinical trial in China, which was carried out in Jiangsu Province in cooperation with the Jiangsu Provincial Center for Disease Control and Prevention.
A statement issued on the INOVIO official website on December 10, 2020 local time shows that the phase II clinical trial of INO-4800 in China has completed the first subject vaccination. This clinical trial is expected to include approximately 640 18-year-olds And above subjects.
“It is expected that in the first quarter of this year, we will be able to start Phase 3 clinical trials.” Recently, Liu Xiaoyan said in an interview with The Paper.
The aforementioned INOVIO statement issued on December 10 last year stated that the Phase 2 clinical trial of INO-4800 in China and the first Phase 2/3 clinical trial conducted in the United States are independent of each other. Liu Xiaoyan introduced that Ai Diweixin and INOVIO jointly developed this COVID-19 DNA vaccine, but each applied for and carried out clinical trials in China and the United States, so phase 1 and phase 2 clinical trials were carried out independently.
On December 23, 2020 local time, the authoritative medical journal “The Lancet” sub-issue “Clinical Medicine” (“EClincalMedicine”) published the results of the Phase 1 clinical trial of INO-4800 in the United States. The paper is “New coronavirus DNA Vaccine INO- The safety and immunogenicity of 4800: A preliminary report of an open-label Phase 1 clinical trial (“safety and immunogenicity of INO-4800 DNA vaccine against SARS-CoV-2: A preliminary report of an open-label, Phase 1 clinical trial”).
The test results show that INO-4800 has good safety and tolerability, and by triggering cellular or (and) humoral immune responses, it is immunogenic in 100% (38/38) of the subjects.
In particular, the research team wrote that only Grade 1 (mild) adverse events (mainly local reactions at the injection site) were recorded in the trial, which has advantages over the existing approved vaccines. For a successful new coronavirus vaccine, safety is very important, which can support the extensive research of INO-4800 in high-risk populations who are more susceptible to complications of new coronavirus infection, such as the elderly and patients with comorbidities.
Let DNA “drill” into the cell
According to a previous report by Shanghai Science and Technology News, the signing ceremony of the cooperation agreement between the National Health Commission/Key Laboratory of the Ministry of Education of Medical Molecular Virology and Ai Di Weixin to strengthen the scientific and technological support of the COVID-19 DNA vaccine was held in June 2020. At that time, the Chinese Academy of Engineering Academician Wen Yumei said that Ai Diweixin’s COVID-19 DNA vaccine has two innovations: First, when constructing DNA, the design of ODN (oligonucleotide) makes the DNA vaccine effective in both cellular and humoral immunity. ; Second, because of the application of electroporation devices, the delivery efficiency of DNA in the human body has been improved.
The electroporation device refers to the CELLECTRA® electroporation device. Liu Xiaoyan introduced that the current phase II clinical trial of INO-4800 in the Jiangsu Provincial Center for Disease Control and Prevention is to use injections and then use the CELLECTRA® electrotransmitter. The CELLECTRA® electrotransmitter is an electrical pulse meter, which is a medical device. After the DNA vaccine has been injected, the electroconverter can quickly complete the delivery of the DNA vaccine.
Liu Xiaoyan introduced that the working principle of the electroporator is to use instantaneous current to physically open the cell membrane. Some holes will appear in the cell membrane momentarily, allowing the DNA plasmid to enter the cell through the holes, thereby greatly improving the delivery efficiency of the DNA vaccine. When the instantaneous current ends, the hole in the cell membrane is closed.
“Currently DNA vaccine-related clinical trials generally use electroporation devices, which are more common. In addition to preventive vaccine products, there are also therapeutic varieties, including cancer vaccines, which will use electroporation devices.” Liu Xiaoyan introduced.
Regarding the introduction of electroporation technology, the article “Research Progress in DNA Vaccine Against Novel Coronavirus” published on November 15, 2020 in the “Chinese Journal of New Drugs” has a detailed introduction. The article stated that in the early stages of DNA vaccine development, after intramuscular and subcutaneous injection, the plasmid DNA is captured by muscle cells and monocytes for antigen expression.
However, naked DNA is easily degraded by deoxyribonuclease (DNase) and lysosome, making it difficult to enter cells, resulting in a very limited expression level of these DNA vaccines, resulting in low immunogenicity.
In recent years, some strategies have emerged to improve the immunogenicity of DNA vaccines, including improvements in delivery routes, and the application of electrical pulses (electroporation) is one of them.
The article stated that the delivery of DNA vaccines through an electric pulse meter can increase the level of DNA vaccines entering animal cells, leading to more antigen expression. The delivery experiment with the introduction of electric pulses proved that the level of DNA vaccines entering muscle cells increased significantly and produced thousands of times the effect of gene expression.
At present, the electric pulse meter has been widely used in various clinical trials, which not only greatly improves the efficiency of DNA vaccine immune activation, but also proves that it has little impact on the human body and no serious adverse events have occurred.
Liu Xiaoyan said that DNA vaccines are generally delivered through physical means (electrotransformation technology), and there is no potential safety risk caused by the introduction of new molecules such as liposomes.
Build China’s largest DNA vaccine and plasmid production base
The article “Research Progress in DNA Vaccines Against New Coronavirus” introduced that nucleic acid vaccines have been called “third-generation vaccine technology” in the industry after traditional attenuated vaccines, inactivated vaccines and genetic engineering subunit vaccines, including DNA vaccines and mRNA vaccine.
DNA vaccine originated in the 1990s. In the past 20 years, scientists have conducted a large number of DNA vaccine studies on animals, including fish, mice, chickens, cats, dogs, pigs and monkeys.
So far, a total of 5 animal DNA vaccine products have been on the market, including DNA vaccines to prevent salmon from being infected with infectious hematopoietic necrosis virus, DNA vaccines to treat dog melanoma, and to reduce the morbidity and mortality of sows during delivery. DNA products and avian influenza DNA vaccine approved by the Ministry of Agriculture of China in 2018. But so far, there is still no human DNA vaccine product on the market.
In an interview with The Paper, Liu Xiaoyan said that before the COVID-19 DNA vaccine, China had not been approved for a preventive DNA vaccine into the clinic. “This vaccine may become the first DNA vaccine approved for marketing in history, and it marks that China has reached the world’s most advanced level in the field of nucleic acid vaccines.” Liu Xiaoyan said.
According to previous reports from Shanghai Science and Technology News, the domestic phase I clinical study of the vaccine was carried out in Huashan Hospital. Zhang Wenhong, director of the Department of Infectious Diseases at Huashan Hospital, was one of the investigators who applied for the clinical trial.
In Zhang Wenhong’s view, this cooperation is meaningful and not only limited to the development and production of the COVID-19 vaccine, because after the COVID-19 epidemic, there may be other infectious disease challenges.
“In China, we must not only carry out vaccine research, but also establish a platform for vaccine research and development. If we encounter epidemics caused by similar viruses in the future, we can respond quickly. After the COVID-19 nucleic acid vaccine enters clinical trials, its safety in humans The sex and effectiveness data will also lay a solid foundation for the future development of nucleic acid vaccines.”
In Liu Xiaoyan’s view, the establishment of a nucleic acid vaccine technology platform is of great significance. “Especially in response to emergencies of public health or biosafety, the nucleic acid vaccine technology platform can effectively provide rapid response technical support and guarantees. Once the nucleic acid vaccine technology platform is determined, the production process and quality control system can be universal and reproducible. It does not change due to the genetic changes of the antigen.
Therefore, the nucleic acid vaccine platform technology CMC has a higher degree of standardization, which can reduce a large amount of pre-clinical work and is suitable for the rapid development of new products. “
While carrying out clinical research, the capacity building of the COVID-19 DNA vaccine is also advancing simultaneously. At present, Ai Diweixin is building China’s largest DNA vaccine and plasmid production base in Village B. The industrialization project of the COVID-19 DNA vaccine is being constructed in phases. The first phase is designed to produce 20 million doses per year, and the second phase is designed to produce 1 100 million doses/year, as of January 2021, the first phase of construction is nearing completion.
(source:internet, reference only)
Disclaimer of medicaltrend.org



