Coronavirus mutations affect the effectiveness of COVID-19 vaccine?
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Coronavirus mutations affect the effectiveness of COVID-19 vaccine?
Coronavirus mutations affect the effectiveness of COVID-19 vaccine? According to Overseas Network, the Public Health Service of England recently released a report stating that a new mutation was found in a sample of a rapidly spreading new coronavirus in the UK. Scientists say that this mutation may evade the protective effect of antibodies, thereby affecting the effectiveness of the vaccine.

Some experts also pointed out that it is too early to infer a major shift in the development trajectory of the UK and global epidemics.
Concerns about the weakening of the vaccine’s effectiveness due to the mutation of the new coronavirus has always caused widespread concern and discussion. According to the Global Times, Moderna and other companies are already preparing to develop a vaccine against the mutation.
On February 1, the Chinese scientific research team published a paper stating that two Chinese COVID-19 vaccines are effective against the mutant strains found in South Africa: Sinopharm and Zhifeilongkema both domestically produced vaccines have maintained high levels of mutant strain 501Y.V2. Protection rate.
01 New mutations in the mutated new coronavirus
According to overseas network citing US media reports, a report from the Department of Public Health of the United Kingdom on February 1 stated that a new mutation that may make the virus evade antibody protection was found in a sample of a rapidly spreading strain in the UK, named E484K The mutant virus samples previously found in South Africa and Brazil already have this genetic feature.
The report showed that this mutation appeared in at least 11 samples of the British B.1.1.7 strain. In addition, some samples may have completed this mutation independently, rather than spread by a single case.
In an interview with the British Broadcasting Corporation (BBC), Calum Semple, a member of the British government advisory panel, said that E484K is “the most concerned mutation” and “naturally appears” in the mutation process of the British virus.
Paul Bieniasz, a virologist at the Rockefeller University in the United States, pointed out that for several months, the E484K mutation appeared “sporadic” in multiple samples, but until recently, this mutation seemed to make the virus resistant to antibodies. It is easier to spread among the crowd.
There are also reports that, although “viral mutation” sounds scary, in fact, in order to adapt to different hosts, mutation is the “normal” in the process of virus self-replication. Dr. Jonathan Stoyer of the Francis Crick Institute in the United Kingdom said that from a virological point of view, mutations are not accidental, and whether mutations can bring great development advantages to viruses needs further observation.
As for why the new coronavirus has such a mutation, some experts pointed out that this may be because someone was infected with a mutant virus found in South Africa or Brazil and a mutant virus found in the UK. This phenomenon is more common in influenza virus infection, but relatively rare in new coronavirus infection.
For this new discovery, there are also concerns. Lawrence Young, a virologist at the University of Warwick in the United Kingdom, said the mutation was “worrying.” He said the fact that the mutated virus found in the UK seems to mutate again “shows that the virus is likely to be adapting to our immune response.”
He said in a statement that this mutated virus may also reinfect people who have previously been infected with the original version of the new coronavirus, partly because the E484K mutation may weaken the human immune response. This mutation may also affect the duration of the antibody response.
The United Kingdom announced the emergence of a mutant new coronavirus B.1.1.7 in December last year. There is evidence that this virus has a stronger transmission capacity. According to data from the US Centers for Disease Control and Prevention, this mutant virus has now appeared in at least 70 countries around the world.
According to US media reports, the Public Health Service of England recently estimated that the infection rate of this mutant virus is 25% to 40% higher than other forms of new coronavirus. There is also preliminary evidence that this mutation may also lead to an increase in mortality.
Since December last year, there have been 105 cases of infection of the new coronavirus variants reported in South Africa in the UK. In order to curb the spread of this variant, the British health department began on the 2nd to test about 80,000 people from house to house for the new coronavirus in 8 regions.
02 or affect vaccine efficacy
Some health experts said that in addition to being known to be more contagious, this mutated new coronavirus may also develop a certain resistance to the immune protection provided by the vaccine, or is more likely to cause infection again in people who have previously been infected.
“As far as the efficacy of the vaccine is concerned, this does not seem to be good news.” said Joseph Fauver, an associate researcher in epidemiology at Yale University’s School of Public Health. He also pointed out that this new discovery is also worthy of continued monitoring by the United States. “At present, we only found this situation in the United Kingdom. This may be because the relevant research in the United Kingdom is relatively strong.”
On the 1st local time, Anthony Fauci, chief medical adviser to the Biden government and director of the National Institute of Allergy and Infectious Diseases, warned that if the new coronavirus variants dominate the spread in the United States, there will still be patients with new coronavirus pneumonia after recovery. May face the risk of re-infection, and the United States may again experience a surge in the number of confirmed cases.
Although experts say that it is too early to predict whether this discovery will affect the UK and global epidemics, in fact, the inference that the COVID-19 virus mutates and then mutates to reduce the effectiveness of the vaccine is not groundless. Studies have shown that the E484K mutation may be the key reason for the poor effectiveness of certain vaccines in South Africa.
Most of the early evidence related to mutations comes from laboratory studies, indicating that antibodies do not seem to be able to bind to the spike protein produced by the mutation. A new study found that antibodies produced by vaccinated people are less effective in neutralizing a synthetic virus that contains key mutations in B.1.1.7 and E484K. However, the study still cannot prove how this mutation will affect the possibility of people being infected with the mutant virus.
Novavax recently announced that its vaccine was 89% effective in a phase 3 clinical trial in the UK, but only 60% in another phase 2b study conducted in South Africa. Similarly, in the Phase 3 clinical trial of Johnson & Johnson in the United States, the effectiveness of the COVID-19 vaccine in different countries is also different: 72% in the United States and 57% in South Africa. In these two trials, 90%-95% of cases in South Africa were related to the B.1.351 mutation, which contained the E484K mutation.
According to Reuters, this E484K mutation occurs in the spike protein of the virus. According to reports, experiments have shown that the E484K mutation makes it more difficult for antibodies to catch the virus and prevent it from entering the cell.
The new coronavirus vaccines jointly developed by American biotechnology company Moderna and Pfizer Pharmaceuticals and German BioNTech are all messenger ribonucleic acid (mRNA) vaccines that work against the spike protein of the new coronavirus.
03 China’s two COVID-19 vaccines are effective against the variants found in South Africa
On February 1, Huang Baoying, Key Laboratory of Biosafety of the National Institute of Disease Control and Prevention of the NHC, Gao Fu of the Chinese Center for Disease Control and Prevention, published an article on the preprint website bioRxiv stating that the mutant strains found in South Africa cannot escape targeting S protein (ZF2001) Or the immune protection induced by the whole virus (BBIBP-corV) vaccine.
BBIBP-CorV is an inactivated COVID-19 vaccine developed by the Beijing Institute of Biological Products. ZF2001 is a ZF2001 recombinant protein subunit COVID-19 vaccine jointly developed by Zhifei and the Institute of Microbiology, Chinese Academy of Sciences. The results of the study showed that although the neutralizing effect of the sera of these two vaccines against South African mutant strains slightly decreased, the decrease was much lower than that of the plasma and mRNA vaccines of recovered patients, and most of the neutralizing activity was still retained. These two vaccines still have a protective effect on the South African mutant strain.
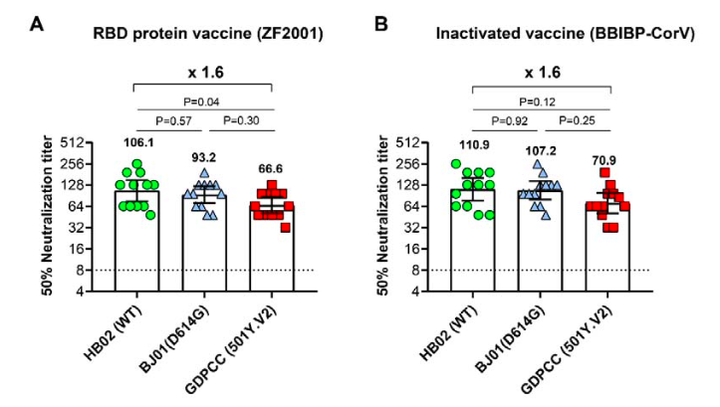
According to the article, the researchers selected 12 serum samples from clinical trial participants for each vaccine. Whether it was ZF2001 or BBIBP-corV subjects, the 12 serum samples basically retained the neutralizing effect of the South African mutant strain. Compared with the titers of the new coronavirus strain WT or D614G, the geometric mean titers (GMTs) decreased by 1.6 times.
This data is significantly better than the previously reported reduction of more than 10-fold in plasma during the recovery period, and also better than the 6-fold reduction in antiserum from mRNA vaccine recipients.
Last month, a study conducted by Moderna and the National Institutes of Health showed that the neutralizing titer of the mRNA-1273 vaccine against the previous mutant strain and the British mutant strain did not change significantly, but the neutralizing titer against the South African mutant strain was reduced. Six times.
On January 29, Johnson & Johnson announced that the test results of its new vaccine in the United States showed that the effectiveness of preventing moderate or severe COVID-19 patients was 72%, compared with 66% in Latin America and South Africa. And 57%.
Recently, the clinical data released by Novavacs showed that the effectiveness of the vaccine reached 89.3%. However, the test also found that the vaccine was 85.6% effective against a variant called B.1.1.7 where it was first discovered in the UK; its effectiveness against the variant virus (B.1.351) found in South Africa dropped to 49%.
In response to viral mutations, Moderna recently announced its efforts to develop an enhanced needle to resist the variants found in South Africa. According to reports, BioNTech, which is cooperating with Pfizer, is also considering developing an improved vaccine.
04 Many problems in vaccination in various countries
Reuters pointed out that the mutation of the mutated virus reported by the United Kingdom highlights the complexity of epidemic prevention and control, even in the context of vaccine launches and blockades by countries. However, given the rapid spread of mutant viruses, active testing, compliance with prevention and control guidelines, and rapid introduction of vaccines are more important than ever.
“We need to vaccinate as many people as possible as soon as possible.” Fauci previously said, “Although the protection of these mutant viruses is weakened, there is still enough protection to prevent patients from suffering from serious diseases, as well as hospitalization and death. “
The Centers for Disease Control and Prevention (CDC) reported on the 2nd local time that 26,440,836 people in the United States have received the first or more doses of the COVID-19vaccine, exceeding the number of confirmed COVID-19 in the United States. In order to further accelerate the vaccination plan, the US federal government plans to provide vaccines directly to pharmacies nationwide on the 11th to provide people with more vaccination sites.
However, vaccination in the United States continues to cause controversy.
As there is no unified guiding principle for the vaccination plan of each state, all regions have decided on their own who can give priority to vaccination. Such a complicated system has prompted the emergence of “vaccine travel”. Many Americans have vaccinated across states in order to be vaccinated in advance. There are even foreign nationals who travel to the United States for vaccinations.
“Vaccine travel” has caused controversy, and Americans worry that this is creating inequality, which in turn will affect the United States’ national vaccination plan.
Ferguson, a medical ethics scholar at New York University’s Grossman School of Medicine, said that vaccination involves appointments, syringes, and human resources. The federal government generally distributes vaccines and formulates strategies based on the local population. Therefore, when someone is vaccinated across cities, states, or even transnational countries , It will disrupt these arrangements.
On the other hand, a few days ago, a hospital in Bellevue, Washington, USA sent emails to about 100 VIP donors inviting them to register for the COVID-19 vaccination. Washington State Governor Insley said that this is “not the right way” and we need to give everyone a fair chance of vaccination. The hospital has apologized for the matter.
In addition, New York City Mayor Desiree admitted on January 31 that there have been serious racial differences in vaccinations in New York. The number of vaccinated African and Hispanic residents is much lower than that of whites. New York vaccinated whites accounted for 48%, Latinos accounted for 15%, Asians accounted for 15%, and African Americans accounted for 11%. Among vaccinated residents 65 and older, ethnic differences are even more pronounced: Of the approximately 125,000 vaccinated New Yorkers, only 9% are black.
On the 1st local time, the CDC reported that among the first batch of people in the United States to receive the COVID-19 vaccine, white women over 50 years old accounted for the majority. The report highlights racial and ethnic inequality among the people most affected by the COVID-19 pandemic.
According to the report, among the nearly 13 million people who received at least the first dose of the vaccine between mid-December 2020 and mid-January 2021, 63% were women and 55% were over 50 years old. Only about half of them have access to information about race, and 60% of them are white. It is unclear why the information was lost. But the highest risk of infection is African Americans, Hispanics, etc.
On the 1st, Dr. Marcela, the chair of the US government’s COVID-19 health and equity working group, said at a White House press conference: “As of January 30, we have missed 47% of ethnic data…no data guidance. We cannot ensure a fair vaccination plan.”
05 EU vaccine shortage
On the other hand, the vaccine supply problem has also triggered political friction in Europe.
Due to production problems in the Belgian factory, AstraZeneca, the manufacturer of Oxford vaccines, proposed that it will reduce its supply of vaccines to the EU by 60% in the first quarter. This means that during this period the EU will only get about 31 million doses of Oxford vaccine.
This statement triggered a vaccine battle between the EU and the UK. Over the past week, the European Union has successively launched public opinion wars, legal wars, and border wars, requiring vaccine manufacturers to give priority to vaccines.
When AstraZeneca stated that it could not guarantee the supply, the Oxford vaccine had not been approved for use by the European Union. However, the European Union is the first to launch a public opinion war. The President of the European Commission von der Lein strongly stated that according to the signed contract, AstraZeneca is obliged to supply vaccines produced in the UK plant to the EU.
AstraZeneca responded that the contract only mentions trying its best to provide vaccines as soon as possible. In an interview with the media, its French CEO Pascal Soriot said grievously that the team has been working hard to ensure supply, but “we first signed a contract with the United Kingdom, and the United Kingdom said’you supply us first.’ It’s fair.” Vaccines produced in the UK factory must be supplied to the UK first and cannot fill this gap.
EU Health Commissioner Stella Kyriakides insisted: “We reject the logic of first-come, first-served. This may work in a butcher shop but not in a procurement contract.”
Two weeks ago, Klaus Chichutech, the head of the German vaccine regulatory agency, also praised Oxford Vaccines as “excellent and outstanding.” But on January 25, the German media suddenly claimed that the Oxford vaccine is only 8% effective among people over 65. On January 28, the German Vaccine Board officially announced that it is not recommended to vaccinate people over 65 years of age with the Oxford vaccine, because the vaccine lacks effectiveness data at this age.
The embarrassing thing is that a day later, the European Medicines Agency (EMA) officially approved the Oxford vaccine to be used in the European Union and can be vaccinated to adults over 18 years of age. The reason is that although there is no sufficient data for people 55 years and older (only 12% of those participating in the vaccine experiment are over 55 years old), researchers have observed that the vaccine can stimulate an immune response in this age group.

After the soft public opinion battle, there is a tougher legal battle. The governments of Italy and Latvia threatened to take legal measures to sue related companies. On January 29, the EU also released the contract despite the confidentiality clause, which indeed showed that the EU suppliers included AstraZeneca’s UK factory. But it is true that as AstraZeneca said before, the company only needs to “make the most reasonable efforts” to supply, and it did not mention the specific supply time and quantity of the British factory. As far as the contract text is concerned, it is unfounded to force AstraZeneca to transfer goods from the UK through legal means, and even to hold it accountable.
On the same day, the European Union also issued a vaccine export review order, which further escalated the war. The review order stipulates that if vaccines produced in the EU are to be exported to areas outside the EU, they must be declared to the EU authorities. They point to the Pfizer vaccine ordered by the United Kingdom and produced in Belgium. It shows that if you don’t give me the vaccine, I will hold it. The battle of your vaccine.
What caused even greater controversy was that that evening, the European Union initiated the terms of the Brexit agreement, including Northern Ireland, which is still in the EU customs area, as a COVID-19 vaccine export control area, and even set up border controls between Ireland and Northern Ireland to prevent The COVID-19 vaccine flows into Northern Ireland from Ireland.
Maintaining the circulation of people and goods between South and Northern Ireland was the key to resolving the historical problems of Northern Ireland. The absence of a hard border between the two places was also a magic weapon used by the EU to beat the UK many times in the Brexit negotiations. But this time, the EU But the first to step on this red line. What is even more shocking is that the Irish government revealed that the European Union did not even consult their opinions when making this decision, and that it was suspected of infringing on Ireland’s national sovereignty. The local leader of Northern Ireland, Coster, bluntly called the EU’s actions “hostile acts.”
WHO Director-General Dr. Tan Desai pointed out that this vaccine nationalist approach may lead to a slow return to normal society.
Condemned by many parties, the EU withdrew the above decision a few hours later. On the evening of January 30, EU President Von der Lein stated that he had reached an agreement with the British Prime Minister that when the company is fulfilling its contractual responsibilities, EU countries should not restrict vaccine exports. AstraZeneca also promptly stated that it would coordinate the supply of more than 9 million doses.
So far, the European Union’s rush for vaccines has temporarily ceased, but it has completely exposed the shortcomings of vaccine supply. In addition to the Oxford vaccine, Moderna in the United States will also cut its supply. Many places in Spain and France had to suspend vaccination. Currently, only 4.2% of people in the European Union have been vaccinated, while the UK has reached 13%, which is more than four times higher than Germany’s second place in Europe. This does not match the EU’s economic and technological strength. For this reason, the EU has to make a gesture of attacking from all sides to explain to the people.
It’s just that this performance response has little effect. In fact, the European Union should have been working hard to help AstraZeneca’s Belgian factory solve the problem of filtering in the sub-packaging link that restricts production. It can even seek joint production like French pharmaceutical company Sanofi, which assists in the production of Pfizer vaccines after being authorized. Precious time is wasted on unorganized teeth and claws.
The greater test is yet to come. The shortcomings of the EU’s deliberative mechanism are thoroughly exposed in the fight against the epidemic where time is life, and if it continues to spread, it may trigger a crisis of “disintegration”. This is a big test for the EU.
At the beginning of the epidemic last year, EU countries lacked coordination and even intercepted materials from other countries, forcing the EU president to publicly apologize to Italy and other countries. Later, the European Union successfully coordinated 37 countries (27 EU countries and 10 neighboring countries) to buy masks and ventilators, only to get back to the game.
Originally, the EU hoped that the vaccine procurement plan could become a symbol of EU unity and strength. But vaccines are not the same as masks. In order to fight for time, vaccines are ordered while researching. The EU member states have to pick and choose among the many candidate vaccines, and only start negotiating the order after reaching a unified opinion, which has been very time consuming. Three months after the UK and AstraZeneca signed an agreement to purchase 100 million doses of Oxford vaccines, the EU coordinated the purchase intentions of each EU country and signed an agreement with AstraZeneca to purchase 300 million doses of vaccines.
As AstraZeneca said, there are also problems in the British factories, just because the UK orders are placed early and production is early, so there is time to deal with them. However, delayed orders, delayed approvals, and delayed delivery have made EU people impatient.
Seeing that the EU is unreliable, some countries have already done it alone. At present, one million doses of China’s inactivated vaccine have arrived in Serbia, and Hungary has officially approved the use of the vaccine developed by China National Pharmaceutical Group.
The UK, which has already left the European Union, took the opportunity to step on it and kept showing how much its vaccination is ahead of the EU. Prime Minister Johnson also said on January 27 that “it would be a pity if the UK stayed in the EU to participate in the EU’s vaccination program.”
The cost of coordination is high, and the timing of repeated delays has caused direct losses to EU countries, but lives. Will it become common practice to go it alone? This is the biggest test for the EU. Whether we can learn from this and reform ourselves depends on the EU’s next response. Otherwise, even if the vaccine shortage passes the barrier, the next step in formulating economic recovery policies will be another difficulty.
(source:internet, reference only)
Disclaimer of medicaltrend.org



