Why is there little news about the therapeutic drugs for COVID-19?
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Why is there little news about the therapeutic drugs for COVID-19?
Why is there little news about the therapeutic drugs for COVID-19? The COVID-19 vaccine is gradually on the market, but why is there little news about the therapeutic drugs?
From the end of 2020 to the present for more than two months, a variety of COVID-19 vaccines have been approved for marketing worldwide, and large-scale vaccination has begun in many countries and regions such as China, the United States, Russia, Brazil, Southeast Asia, and the Middle East, for the global economy and society The restoration of order brought dawn.
After the celebrations around the world, people have forgotten that apart from vaccines, there is another way to deal with the COVID-19 epidemic—therapeutic drugs. Such drugs include both small-molecule chemical drugs and large-molecule biological drugs, usually for the treatment of new coronavirus infections. Under the dazzling aura of the COVID-19 vaccine, the COVID-19 treatment drugs seem to be gloomy. So, why is there little news about COVID-19 treatment drugs? What about its real research and development situation?
The global epidemic situation continues to be severe
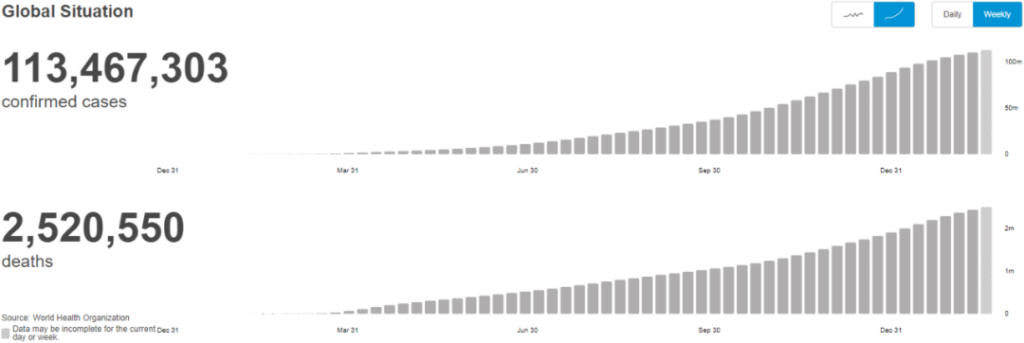
Figure 1. The cumulative number of confirmed and dead cases of new coronavirus pneumonia worldwide (as of February 28, 2021)
Image source: World Health Organization, Zhongkang Industrial Capital Research Center
According to statistics from the World Health Organization (WHO), from December 30, 2019 to February 28, 2021, in a 14-month period, the cumulative number of confirmed cases of new coronavirus pneumonia worldwide exceeded 113 million, of which the cumulative number of deaths Over 2.52 million, the overall case fatality rate is about 2.22%. The cumulative number of confirmed cases of the COVID-19 epidemic globally has not yet peaked, and the situation remains grim.
Among the major epidemic areas in the world, the combined proportions of confirmed cases and deaths in the Americas and Europe are approximately 78% and 82%, respectively. In contrast, the Western Pacific region, including China, Japan and South Korea, has become the region with the most easing epidemic in the world due to early response and appropriate measures. The mortality rate of new coronavirus pneumonia in the six major regions of the world is between 1.54% and 2.53%, which is higher than influenza but lower than SARS and MERS (Middle East Respiratory Syndrome) caused by other coronavirus strains.
COVID-19 vaccine and therapeutic drug application prospects
In the face of the continuing severe nCOVID-19 epidemic, in addition to social isolation and wearing masks for uninfected people, it is recognized that the most effective prevention method is to vaccinate. According to the statistics of the United Nations Population Fund (UNFPA), the global population is 7.795 billion in 2020, so the proportion of the population diagnosed with new coronavirus pneumonia is about 1.46%, and the remaining 98.54% of the population are potential targets for the COVID-19 vaccine. Regardless of the cost of treatment, the number of people applying the COVID-19 vaccine is more than 67 times that of the COVID-19 treatment drug.
In view of the small number of COVID-19 vaccines currently approved and the limited production capacity, it will not be able to meet the vaccination needs of 98.54% of the global population in the short term. The elderly (65 years and older) are people with a high risk of chronic diseases such as high blood pressure, hyperlipidemia, and diabetes. They are prone to develop severe infections after being infected with the COVID-19 virus, which brings a higher risk of death. Therefore, they should be given the highest priority for vaccination. According to UNFPA statistics, the proportion of the global elderly population is 9.3%, so even if only the elderly population is considered, the COVID-19 vaccine application population is more than 6 times the COVID-19 treatment drug.
In addition, it should be noted that the cumulative number of confirmed patients with new coronavirus pneumonia according to WHO statistics is about 113 million. However, according to the severity of new coronavirus pneumonia, it usually recovers within a few days to a few months. The number of patients currently in recovery should be more than that. 113 million less than much. Then, the gap between the number of people applying the COVID-19 vaccine and the COVID-19 treatment drug will further expand.
Looking at the cost, take the first approved COVID-19 treatment drug remdesivir (remdesivir, Veklury) as an example. According to Gilead’s pricing, patients with private insurance pay US$520 per dose and a course of treatment (5 The cost of 6 doses per day is US$3,120; patients with government medical insurance need to pay 75% of private insurance. In addition, remdesivir does not reduce the mortality rate, and its approval by the US FDA is based on the ability to shorten the recovery time of hospitalized patients. The relatively high price of Remdesivir has caused more controversy around the world.
As for the price of the nCOVID-19vaccine, the current vaccine development country has not announced the price. As a public health product, the COVID-19 vaccine should be priced based on cost and obtain less profit. Vaccine purchasers are generally governments of various countries, and they are provided to the public for free or at a low price.
In December 2020, Jiangsu Province released the results of the COVID-19 vaccine procurement. The winning bids for the inactivated COVID-19 vaccines of Kexing Biological and Sinopharm Beijing Research Institute were both RMB 200 per bottle and RMB 400 for the two-shot immunization program. In August 2020, Pfizer and BioNTech revealed that according to the pre-purchase agreement with the U.S. Department of Health and Human Services (HHS) and the Department of Defense, the price of each vaccine is $19.5 and the two-shot immunization program is $39. However, this price does not include the additional costs of cold chain transportation, storage, and injection of vaccines.
The Global Alliance for Vaccines and Immunization (GAVI), the Alliance for Epidemiological Prevention and Innovation (CEPI) and the World Health Organization have established the Global Access Guarantee Mechanism for New coronavirus Vaccines (COVAX) in order to promote the development, production and fair access of COVID-19 vaccines. According to the COVAX survey report, the highest price of the COVID-19 vaccine in rich countries should not exceed US$40 per dose, and it must be cheaper in poor countries. According to this suggested price, the price of the 2-shot immunization procedure should not exceed 80 US dollars (about 560 yuan). Whether it is the purchase price of China’s COVID-19 vaccine, the U.S. negotiated price of Pfizer and BioNTech, or the suggested price of COVAX, they are all well below the price of $3,120 for a course of remdesivir.
Although remdesivir’s higher price will bring disadvantages to market promotion, but it will not break fast. It was the first to obtain an emergency use authorization (EUA) from the US FDA in May 2020, and then it was formally approved in October. Excluding the donated 1.5 million bottles, the sales of Remdesivir in the third quarter were US$873 million, and the surge in hospitalized patients reached US$1.93 billion in the fourth quarter, and sales exceeded US$2.8 billion in more than half a year.
In the period when the COVID-19 vaccine has not yet been approved, Remdesivir almost dominates the market. However, the COVID-19 vaccine has been approved for marketing one after another, and the large-scale use will reduce the incidence rate. After exhausting the stock market, the incremental market for COVID-19 therapeutic drugs such as Redecive will gradually shrink.
In addition, it is also necessary to consider that patients with new coronavirus pneumonia do not have to use remdesivir. At present, the treatment of patients is mainly based on symptomatic treatment, rather than treatment of the cause. The diagnosis and treatment plans issued by China include general treatment (rest, oxygen therapy, etc.), antiviral treatment (previously marketed antiviral drugs), immunotherapy, and traditional Chinese medicine treatment, and these therapies are usually effective. As more effective symptomatic therapies are developed and promoted, the market penetration rate of COVID-19 treatment drugs such as Remdesivir will be further reduced.
Overview of the development of COVID-19 therapeutic drugs
The current COVID-19 treatment drugs, in addition to the FDA officially approved Remdesivir, there are also several neutralizing antibodies that have obtained emergency use authorization (EUA), such as Regeneron’s REGN-COV2 (casirivimab/imdevimab), Regeneron and Jun Bamlanivimab/etesevimab (LY-CoV555/JS016) jointly developed by Shibi. The new coronavirus neutralizing antibody is special and can be used to treat patients with new coronaviruspneumonia. For example, former US President Trump has received REGN-COV2 treatment, and it can also be used for short-term prevention of new coronavirus infection.
According to statistics on public clinical trial databases (ClinicalTrials.gov in the United States and ChiCTR.org.cn in China) in mid-February 2021, there are currently about 200 phase III and phase IV clinical trials of COVID-19 treatment drugs in the world. 36 of these trials will release data before the end of April. These 36 clinical trials include 28 phase III clinical trials, 7 phase IV clinical trials, and 1 master agreement trial (ACTIV-1 IM) for which interim results will be released soon. These 36 clinical trials are about 27 candidate COVID-19 treatment drugs.
The 27 drug candidates include 4 types of drugs: antimicrobial drugs, antithrombotic drugs, immunomodulators, metabolic modulators, and a small number of other drugs. Among them, immunomodulators are the largest category, including 10 drug candidates, such as colchicine, methylprednisolone, infliximab, etc.; antimicrobial drugs and antithrombotic drugs each have 5 drug candidates.
In general, in order to accelerate the speed of research and development, the development strategy of most candidate COVID-19 therapeutic drugs is to expand the indications of mature drugs that have been on the market for many years. There are also a few unlisted drugs, such as cenicriviroc for HIV and mesenchymal liver cells ( MSC) therapy remestemcel-L and so on. It is worth noting that a considerable proportion of clinical trials are initiated by academic institutions, and their funding sources and R&D capabilities are usually limited. Under the impact of the continuous listing of COVID-19 vaccines, many companies and academic institutions may terminate the research and development of COVID-19 treatment drugs.
Conclusion
Around the Spring Festival, Chinese and foreign COVID-19 vaccines have been approved one after another, which has brought good news to the world. It is expected to promote the health of all mankind, restore the global economic and social order, and promote global development on the right track. Although the application of the COVID-19 vaccine has brought a huge impact to the market of COVID-19 therapeutic drugs, the long-term immune effect of the nCOVID-19 vaccine remains to be seen. For patients with immune failure or secondary infection, the COVID-19 treatment drugs will play a major role. Although the vaccine is good, the therapeutic drugs are also an essential part of the prevention and treatment plan. For the health of all mankind, the nCOVID-19 vaccine and COVID-19 treatment drugs are indispensable.
(source:internet, reference only)
Disclaimer of medicaltrend.org



