Analysis of the targets of new drugs on the global market in 2020
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Analysis of the targets of new drugs on the global market in 2020
Analysis of the targets of new drugs on the global market in 2020. In 2020, a total of 61 innovative drugs have been approved in the United States, the European Union and Japan, some of which have innovative biomolecular target mechanisms.
| Drug | Attributes | Target mechanism | Target classification | Indications | Approval |
| Bempedoic acid | Small molecule inhibitor | ATP-citrate synthase (ACLY) | Enzyme | Hypercholesterolemia | FDA,EMA |
| Tazemetostat | Small molecule inhibitor | Histone Lysine N-Methyltransferase EZH2 (EZH2) | Enzyme | Epithelioid sarcoma and follicular lymphoma | FDA |
| Lonafarnib | Small molecule inhibitor | Farnesyltransferase (FPT) | Enzyme | Hutchinson – Guildford Progeria Syndrome (HGPS) and Progeria-like Laminopathy | FDA |
| Cedazuridine | Small molecule inhibitor | Cytidine Dehydrogenase (CDA) | Enzyme | Myelodysplastic syndrome | FDA |
| Remdesivir | Small molecule inhibitor | SARS-CoV-2RNA-dependent RNA polymerase (RdRp) | Enzyme | COVID-19 | FDA, EMA, PMDA |
| Bulevirtide | Peptides | Sodium/bile acid transporter (SLC10A1) | Transporter | Chronic hepatitis D virus infection | EMA |
| Ansuvimab ; atoltivimab , odesivimab , maftivimab | Monoclonal antibody | Ebola virus glycoprotein (EBOV GP) | Viral glycoprotein | Ebola virus infection | FDA |
| Belantamab mafodotin | Antibody Drugs (ADC) | TNF receptor superfamily member 17 (TNFRSF17, BCMA) | Tumor-associated antigen | Multiple myeloma | FDA,EMA |
| Sacituzumab govitecan | ADC | Tumor-related calcium signal sensor 2 (TACSTD2, TROP2) | Tumor-associated antigen | Triple negative breast cancer | FDA |
| Lumasiran | siRNA | Oxyacid oxidase 1 (HAO1) | RNA | Type I hyperoxaluria | FDA,EMA |
| Borofalan(10B) | 10B-labeled BPA for Boron Neutron Capture Therapy (BNCT) | Y+L amino acid transporter 1 (SLC7A7) | Transporter | Head and neck cancer | PMDA |
| Ga 68 PSMA-11 | Imaging agent | Prostate specific antigen (KLK3) | Tumor-associated antigen | Prostate cancer diagnosis | FDA |
| Flortaucipir F 18 | Imaging agent | Microtubule-associated protein tau (MAPT) | Structural skeleton | Alzheimer’s disease (AD) diagnosis | drug |
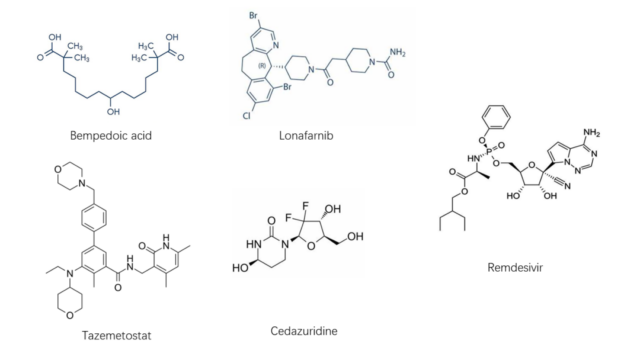
Based on the analysis of literature, here are some of the important drugs and their targets.
Among the targets of the above-mentioned drugs, five (Bempedoic acid, Tazemetostat, Lonafarnib, Cedazuridine, Remdesivir) are enzymes that are inhibited by small molecule drugs, and four are human-derived proteins.
It is worth noting that the target protein of Remdesivir is SARS-CoV-2 RNA-dependent RNA polymerase, which is the first drug approved by the entire FDA to treat COVID-19.
Another viral target is the Ebola virus glycoprotein. For this protein, four innovative monoclonal antibodies (Ansuvimab, atoltivimab, odesivimab, maftivimab) have been approved, three of which are used as a combination therapy, and one can be a single agent.
In addition to small molecules and monoclonal antibodies, three other drug targets are based on therapeutic platforms. Two of them are antibody-drug conjugates (Belantamab mafodotin, Sacituzumab govitecan), which recognize the tumor-specific targets BCMA and TROP2, respectively, to selectively deliver cytotoxic components to tumor cells-the microtubule blocker monomethyl Austenite Bactericin F and topoisomerase I inhibitor SN-38.
The third drug lumasiran is the third approved small interfering RNA therapy, which treats rare disease type 1 hyperoxaluria by reducing the mRNA of oxyacid oxidase 1.
From the perspective of the therapeutic field, the above-mentioned seven innovative target mechanism drugs are used to treat rare diseases, including two family genetic diseases, two viral infections and three cancers.
In addition, there is a boron delivery agent (Borofalan) for tumor boron neutron capture therapy and two (Ga 68 PSMA-11, Flortaucipir F 18) for the diagnosis of prostate cancer and Alzheimer’s disease. The image agent is approved.
(source:internet, reference only)
Disclaimer of medicaltrend.org



