Analysis of the characteristics of new drugs approved by the FDA in 2020
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Analysis of the characteristics of new drugs approved by the FDA in 2020
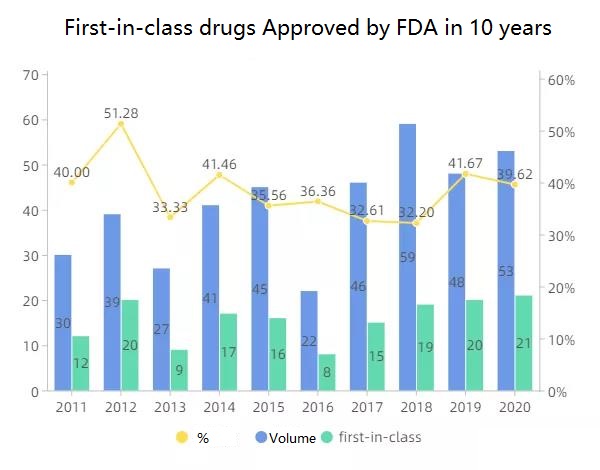
Analysis of the characteristics of new drugs approved by the FDA in 2020. As the proportion of first-in-class drugs in FDA approved drugs has increased year by year, it has revealed important opportunities for the market for this class of drugs. Here, let’s review the structure of 21 representative First-in-class drugs (chemical) in 2020. And its characteristics.
| Serial number | Product name | Common name | Target | Approval type | R&D company | Indications |
| 1 | Blenrep | belantamab mafodotin | B cell maturation antigen (BCMA) antibody coupled to tubulin polymerization inhibitor | P,O,A,B | GlaxoSmithKline | Multiple myeloma |
| 2 | Solitaire | satralizumab | Interleukin-6 (IL-6) monoclonal antibody | O,B | Genentech/Roche | Neuromyelitis optica spectrum disorder |
| 3 | Ga 68 PSMA-11 | ga 68 PSMA-11 | Prostate specific membrane antigen (PSMA) | UCLA and San Francisco | Diagnose prostate cancer | |
| 4 | Imcivree | setmelanotide | Melanocortin-4 receptor (MC4R) agonist | P,O,B | Rhythm Pharmaceuticals | Rare genetic obesity |
| 5 | Inmazeb | atoltivimab / maftivimab / odesivimab-ebgn / REGN-EB3 | Virus surface glycoprotein | P,O,B | Regenerating element | Ebola |
| 6 | Isturisa | osilodrostat | 11β hydroxylase inhibitor | THE | Novartis | Cushing syndrome |
| 7 | Klisyri | tirbanibulin | Microtubule inhibitor | Athenex, Almirall | Actinic keratosis | |
| 8 | Koselugo | selumetinib | MEK1/2 inhibitor | P,O,B | AstraZeneca Merced | Neurofibromas |
| 9 | Monjuvi | tafasitamab-cxix | CD19 monoclonal antibody | P,O,A,B | MorphoSys | B cell lymphoma |
| 10 | Nexletol | bempedoic acid | ATP Citrate Lyase Inhibitor (ACL) | Esperion Therapeutics | reduce cholesterol | |
| 11 | Oxlumo | lumasiran | RNAi of Hydroxy Acid Oxidase 1 (HAO1) | P,O,B | Alnylam | Primary hyperoxalic acid type 1 |
| 12 | Rukobia | fostemsavir | Adhesion inhibitor | P, B | ViiV Healthcare | AIDS |
| 13 | Ropes | flortaucipir F18 | Abnormal tau protein in the brain | S | Eli Lilly | Diagnosing Alzheimer’s disease |
| 14 | Tazverik | tazemetostat | zeste gene enhancer homolog 2 (EZH2) inhibitor | P | Epizyme | Epithelioid sarcoma |
| 15 | Tepezza | teprotumumab-trbw | Insulin-like growth factor-1 receptor (IGF-1R) monoclonal antibody | P,O,B | Horizon Pharma | Thyroid Eye Disease |
| 16 | Trodelvy | sacituzumab govitecan | TROP-2 antigen antibody coupled to topoisomerase I inhibitor | P,A,B | Immunomedics | Triple negative breast cancer |
| 17 | Uplizna | inebilizumab-cdon | CD19 monoclonal antibody | O,B | Viela Bio | Neuromyelitis Optic Disorder |
| 18 | Veklury | remdesivir | RNA-dependent RNA polymerase (RdRp) inhibitor | P | Gilead Sciences | COVID-19 |
| 19 | Winlevi | clascoterone | Androgen receptor (AR) inhibitor | Cassiopea | Acne | |
| 20 | Xeglyze | abametapir | Metalloproteinase inhibitor | Dr. Reddy Labs | Lice disease | |
| 21 | Zokinvy | lonafarnib | Farnesyltransferase inhibitor | P,O,B | Eiger Bio Pharmaceuticals | Progeria and Progeria-like Laminopathy |
P: Priority review; O: Orphan drug; A: Accelerated approval; B: Breakthrough therapy
1. Blenrep
Blenrep is the world’s first approved BCMA therapy drug, and its active ingredient belantamab mafodotin-blmf is an antibody-conjugated drug (ADC). The antibody component is afucosylated IgG1 targeting B cell maturation antigen (BCMA), and the small molecule component is the microtubule inhibitor MMAF. After binding to BCMA, belantamab mafodotin-blmf is internalized and hydrolyzed sequentially to release MMAF. The released MMAF destroys the microtubule network in the cell, leading to cell cycle arrest and apoptosis.
The white blood cell maturation antigen (BCMA) was discovered in 1992 and belongs to the tumor necrosis factor superfamily (TNFRSF17/CD269). It is widely present on the surface of multiple myeloma cells, but has low expression on other normal cells, so it is multiple myeloma And a popular target for other hematological malignancies. Currently, there are at least 43 BCMA therapies in progress in the world, which are mainly divided into three categories: chimeric antigen receptor T cell therapy (CAR-T therapy, Xinji/Bluebird Bio, Novartis, etc.), bispecific antibodies (BsAb , Amgen, etc.) and antibody-conjugated drugs (ADC, GSK, etc.).
2. Enspryng
Enspryng is the first and only drug approved for subcutaneous injection for the treatment of neuromyelitis optica spectrum disorder (NMOSD) with positive anti-aquaporin-4 (AQP4) antibody. The active ingredient Satralizumab is a PH-dependent humanized anti-interleukin 6 receptor (IL-6R) monoclonal antibody. So far, the FDA has approved three drugs for the treatment of NMOSD, which act on different stages of the disease. Satralizumab mainly inhibits the differentiation of B cells into plasma cells to prevent the production of AQP4 autoantibodies, while Uplizna prevents the production of AQP4 autoantibodies by directly eliminating CD19-expressing plasma cells. Soliris mainly reduces brain damage by inhibiting the activated complement system.
3. Ga 68 PSMA-11
Gallium 68 PSMA-11 (Ga 68 PSMA-11) is the first drug used for positron emission tomography (PET) imaging of prostate-specific membrane antigen (PSMA) positive lesions in male patients with prostate cancer. The early symptoms of prostate cancer are not obvious, and it is easy for patients to ignore it. About half of the patients are already at an advanced stage when they are diagnosed. Previous testing methods, such as digital rectal examination, PSA testing, ultrasound, needle biopsy, CT, MRI, and bone scan, have limitations in detecting prostate cancer lesions.
Prostate-specific membrane antigen (PSMA) is significantly up-regulated in most prostate cancer cells, and is closely related to the invasion, metastasis, staging, grading, castration therapy resistance, and biochemical recurrence of the disease. It is an ideal diagnostic marker for prostate cancer Things. The high sensitivity and high specificity of Ga 68 PSMA-11 make accurate diagnosis of prostate cancer a reality.
4. Imcivree
Imcivree is the world’s first drug approved for the treatment of obesity against gene defects. Its active ingredient setmelanotide is an oligopeptide melanocortin 4 receptor (MC4R) agonist. In the past, people always attributed obesity to bad habits, but a small number of people are caused by genetic mutations.
MC4R is a part of the key biological pathways that the body independently regulates energy expenditure and appetite. Certain genetic mutations may impair the function of the MC4R pathway and cause excessive appetite. Imcivree activates MC4R to activate the areas of the brain that regulate appetite and satiety, and control the patient’s weight.
5. Inmazeb
Inmazeb is the world’s first drug approved to treat Ebola infection. The drug is a triple antibody cocktail therapy, the active ingredient is a mixture of 3 fully human IgG1 monoclonal antibodies (atoltivimab, maftivimab, odesivimab-ebgn). These three types of IgG1 have similar structures. They neutralize the virus by targeting glycoproteins at different sites on the surface of the Ebola virus, blocking the attachment and invasion of the virus and recruiting immune cells.
6. Isturisa
Cushing syndrome is a rare disease in which cortisol increases due to multiple reasons. The first choice for most patients with Cushing’s disease is surgery, but not all patients are eligible for surgery, and surgery is not always effective. In the past, there were few choices of drugs used to lower cortisol levels, and these drugs either had limited efficacy or had too many side effects. Isturisa is the first oral 11β-hydroxylase (CYP11B1) inhibitor approved by the FDA for the treatment of Cushing’s syndrome.
The active ingredient Osilodrostat directly solves the cortisol overdose by blocking the last step of cortisol synthesis. First-line drugs are used to treat adult patients with Cushing’s disease who cannot undergo pituitary surgery or whose symptoms persist after surgery.
7. Klisyri
Klisyri is an ointment used to treat actinic keratosis (AK) of the face or scalp. Its active ingredient, tirbanibulin, is a microtubule inhibitor that induces proliferation of cells by inhibiting the polymerization of microtubules. Apoptosis. Due to environmental factors such as ozone depletion and excessive sun exposure, AK will be a huge and growing market, and it is expected that the market value will reach US$1.558 billion by 2026.
In the past, the main treatment for primary AK was destructive cryotherapy, and Klisyri ointment not only has good clinical safety, but also can be effective within 5 days of treatment. It is expected to produce huge commercial value in the pharmaceutical and cosmetic industries.
8. Koselugo
Koselugo is the first drug approved for the treatment of neurofibromatosis type 1 (NF1). Its active ingredient, selumetinib, can selectively inhibit MEK1 and MEK2, thereby returning the dysregulated RAS signaling pathway to normal and alleviating the patient’s condition. . NF1 is a rare and incurable genetic disease with an incidence rate of 1/3000-4000 people. It is caused by mutations in the NF1 gene that synthesizes neurofibroma protein. This gene mutation can disrupt the RAS/MAPK signaling pathway, and then Cause tumor growth. In addition, Koselugo is not only the first drug to treat NF1, but also the first drug to treat this rare disease that is debilitating early in life.
9. Monjuvi
Monjuvi is the first second-line therapy approved by the FDA for adult patients with diffuse large B-cell lymphoma (DLBCL) during or after first-line treatment. The active ingredient tafasitamab is a new humanized Fc enhancement targeting CD19 Monoclonal antibodies, through antibody-dependent cell-mediated cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP), significantly kill B cell malignant tumor cells. Monjuvi not only has the same curative effect as the two previously approved CAR-T therapies (Kymriah and Yescarta), but also has more advantages over CAR-T therapy in terms of medication and price, and has huge market potential.
10. Nexletol
Nexletol is the first oral, once-daily, non-statin, low-density lipoprotein cholesterol (LDL-C)-lowering drug approved by the FDA in the past 20 years. Its active ingredient, bempedoic acid, is a precursor of dicarboxylic acids. The drug is activated in the body by the very long-chain acetyl-CoA synthase 1 (ACSVL1) and converted into an ATP citrate lyase (ACL) inhibitor, which reduces LDL-C by inhibiting the synthesis of cholesterol in the liver.
Although statins are currently used as the standard treatment for lowering cholesterol, not all patients can tolerate statins, and some patients may not necessarily reach the standard for LDL-C levels when they reach the maximum tolerable value of statins. The marketing of this drug with a new lipid-lowering mechanism provides an important treatment option for patients.
11. Oxlumo
Oxlumo is the world’s first drug for the treatment of primary hyperoxaluria type 1 (PH1). Its active ingredient is lumasiran, a small molecule interfering ribonucleic acid (siRNA) that targets the liver encoding glycolate oxidase (glycolate). oxidase, GO) HAO1 gene mRNA silences HAO1 and consumes GO, thereby reducing the level of oxalate in plasma and urine and preventing the progression of PH1 disease.
PH1 is a rare autosomal recessive genetic disease, caused by excessive oxalic acid production leading to kidney failure. PH1 usually develops in childhood and requires immediate and effective intervention. Advanced patients have no choice other than frequent kidney dialysis or combined liver and kidney organ transplantation. The approval of Oxlumo on the market will have an important clinical impact on PH1 patients.
12. Rukobia
Rukobia is an anti-HIV drug with a new mechanism of action to overcome multi-drug resistance. The active ingredient fostemsavir is a prodrug. After oral administration, it can be transformed into an adhesion inhibitor temsavir in the body, which is recognized by the glycoprotein 120 (gp120) subunit on the surface of the virus. And combined, block the interaction of the virus with the host immune system CD4+ T cells or other immune cells, thereby preventing the virus from infecting the host cells. Since Rukobia is the first step (attachment) that targets the HIV virus cycle, it has no cross-resistance with other antiretroviral (ARV) drugs that have been approved. Drug suppression and maintenance of viral suppression provide a new treatment option for multi-drug resistant HIV-1 adults infected.
13. Tauvid
The neuropathological diagnosis of Alzheimer (AD) requires proof of the presence of both β-amyloid neuritis plaques and tau neurofibrillary tangles in the brain(Tau NFT). Tauvid is the first and only diagnostic agent approved for imaging tau-NFT in the brain.
The FDA has previously approved Amyvid to prove the presence of beta amyloid plaques. Based on the combination of these two diagnostic reagents, AD is expected to achieve more accurate early detection.
14. Tazverik
Tazverik is the first and only drug specifically designed for the treatment of epithelioid sarcoma (ES). Its active ingredient, tazemetostat, is an epigenetic drug, and it is the first and only approved zeste gene enhancer. Source 2 (EZH2) inhibitor is also the first histone methyltransferase (HMT) inhibitor to be marketed. Abnormal activation of EZH2 usually leads to dysregulation of genes that control cell proliferation, leading to unrestricted and rapid growth of non-Hodgkin’s lymphoma (NHL) and other solid tumor cells.
Inhibition of EZH2 activity is expected to play in multiple cancer types Anti-tumor effect, tazemetostat is currently being developed for various types of hematological malignancies (non-Hodgkin’s lymphoma: relapsed or refractory diffuse large B-cell lymphoma [DLBCL], follicular lymphoma [ FL]) and genetically defined solid tumors (epithelioid sarcoma, synovial sarcoma, INI1-negative tumors, castration-resistant prostate cancer, platinum-resistant solid tumors, etc.).
15. Tepezza
Tepezza is the first and only drug approved for the treatment of Thyroid Eye Disease (TED). The active ingredient teprotumumab is a fully humanized insulin-like growth factor 1 receptor (IGF-1R) monoclonal antibody. It binds to IGF-1R and blocks its activation and signal transduction, reduces inflammation and prevents cell overgrowth. TED is a rare and progressive autoimmune disease.
The human body attacks its own orbital fibroblasts overexpressing IGF-1R, causing inflammation of the muscles and fatty tissue behind the eyes, causing the eyes to push forward and bulge outward. Long-term accumulation of this irreversible damage can even lead to blindness.
16. Trodelvy
Trodelvy is the first ADC drug in the field of metastatic triple-negative breast cancer (mTNBC) and the first ADC drug that targets Trop-2 on the market. The active ingredient, sacituzumab govitecan, is formed by coupling a humanized IgG1 antibody targeting TROP-2 antigen to the metabolically active product SN-38 of the chemotherapy drug irinotecan (a topoisomerase I inhibitor), which targets TROP -2 This cell surface glycoprotein, which is expressed in more than 90% of TNBC, specifically kills tumor cells.
TNBC is an aggressive cancer with a poor prognosis. In addition to traditional chemotherapy, treatment options are extremely limited. Trodelvy has the potential to become a standard care drug for the treatment of TNBC.
17. Uplizna
Uplizna is the second drug approved for the treatment of NMOSD (previously introduced), and is the first and only B cell depleting agent in this disease field. The active ingredient inebizumab is a humanized, affinity-optimized, non-fucosylated IgG1 monoclonal antibody that targets CD19.
This drug can quickly clear CD19-expressing B cells from the blood circulation, thereby reducing antigen presentation and promoting inflammation Factor to relieve the patient’s symptoms. The three drugs approved by NMOSD act on different stages of the disease.
Enspryng mainly inhibits the differentiation of B cells into plasma cells to prevent the production of AQP4 autoantibodies, while Uplizna prevents the production of AQP4 autoantibodies by directly eliminating CD19-expressing plasma cells. Soliris mainly Reduce brain damage by inhibiting the activated complement system.
18. Veklury
Veklury is the first and only drug approved by the FDA to treat COVID-19. The active ingredient remdesivir is a nucleotide analogue that has broad-spectrum antiviral activity in animals and in vitro, and can fight against a variety of emerging viral pathogens, including Ebola virus, atypical pneumonia virus, and Marburg virus , Middle East Respiratory Syndrome and SARS-CoV-2 virus that causes new coronavirus pneumonia, etc. Unfortunately, clinical trials have shown that this drug is only effective in shortening the recovery time of patients, but the improvement in patient mortality is not significant.
19. Winlevi
Winlevi is the first acne drug with a new mechanism of action (MOA) approved by the FDA in the nearly 40 years since 1980. It is also the first safe and effective local androgen inhibitor therapy without systemic side effects. The active ingredient clascoterone is a topical androgen receptor inhibitor, which can penetrate the skin to reach the androgen receptor in the sebaceous glands and hair follicles, inhibiting sebum secretion and inflammation. Unlike previous oral hormones used to treat acne, Winlevi can be used in both male and female patients.
20. Xeglyze
Xeglyze is the first medicine approved for the treatment of head lice. The active ingredient abametapir is a metalloproteinase inhibitor that can inhibit head lice from incubating eggs and affect the development of eggs and the survival of adults.
21. Zokinvy
Zokinvy is the world’s first drug approved for the treatment of progeria (Hutchinson-Gilford Progeria Syndrome HGPS) and progeroid laminopathies (PL). Its active ingredient is lonafarni, an oral farnesyl transferase inhibitor (Farnesyltransferase Inhibitor, FIT), which can block the farnesylation of progeria proteins and prevent defective proteases or protease-like accumulation.
Progeria is an extremely rare and fatal genetic disease that accelerates the aging of children. Gene mutations produce progerin with abnormal farnesylation. Progeria-like laminopathy is caused by gene mutations to produce farnesylated proteins that are different from progeria, which can lead to overlapping but different disease manifestations with progeria.
Zokinvy can effectively delay the aging and lifespan of patients with these two diseases.
(source:internet, reference only)
Disclaimer of medicaltrend.org



