Roche New Prot-2+1 TCB Antibody
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Roche New Prot-2+1 TCB Antibody
Roche New Prot-2+1 TCB Antibody. Glofitamab is a T cell bispecific antibody targeting CD20XCD3. The structure of the antibody is “2+1” (CD20-TCB), that is, there are two CD20 binding sites and one CD3 binding site.
Phase I clinical data show that the antibody has a strong and powerful effect on B-NHL, and has a longer-lasting relief and controllable safety.
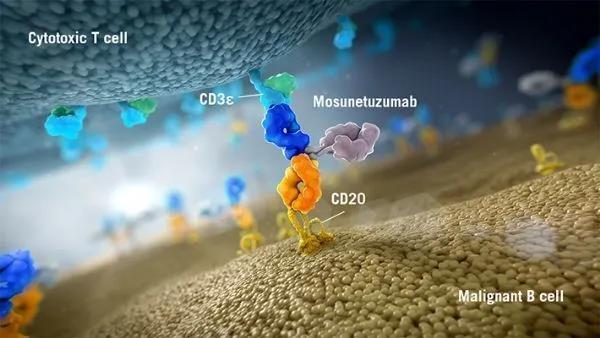
The success of CD20-TCB has inspired researchers to explore more extensively. Based on the structure of this antibody, Roche has also constructed related antibodies for the treatment of solid tumors, such as CEA-TCB (RO6958688) and FOLR1-TCB.
CEA-TCB preclinical data show that antibodies can specifically bind to tumor cells with high CEA expression (CEA antigen expression is greater than 10,000), and activate T cells to kill tumor cells, but cannot bind to normal cells expressed by CEA (CEA antigen The expression is less than 10000).
At present, the antibody has completed the first phase of clinical trials and has certain curative effects. However, in the preclinical experiments of FOLR1-TCB, a lower antibody dose can trigger on-target off-tumor toxic side effects. In order to solve the on-target off-tumor effect of TCB antibodies, Roche made improvements on the basis of TCB. Invented Prot-TCB.
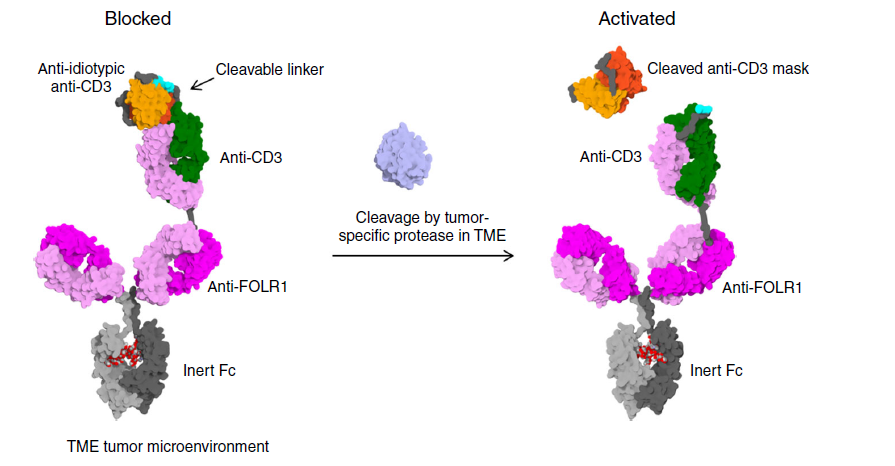
Figure 1 Prot-TCB mechanism of action
Prot-TCB design ideas
Unlike the original TCB, Prot-TCB puts the Fab that binds the CD3 antigen on the top of the long arm, and at the same time fused the Fab with the single-chain antibody scFv (Anti-idiotypic anti-CD3) against this Fab.
The connection between Fab and scFv is through a specific enzyme cut site. In tumors, this site is specifically cleaved by enzymes and exposes the CD3 binding site, which activates T cells to kill tumor cells. In normal tissues, this site The spot cannot be cleaved, so the antibody cannot bind to the CD3 antigen to activate T cells.
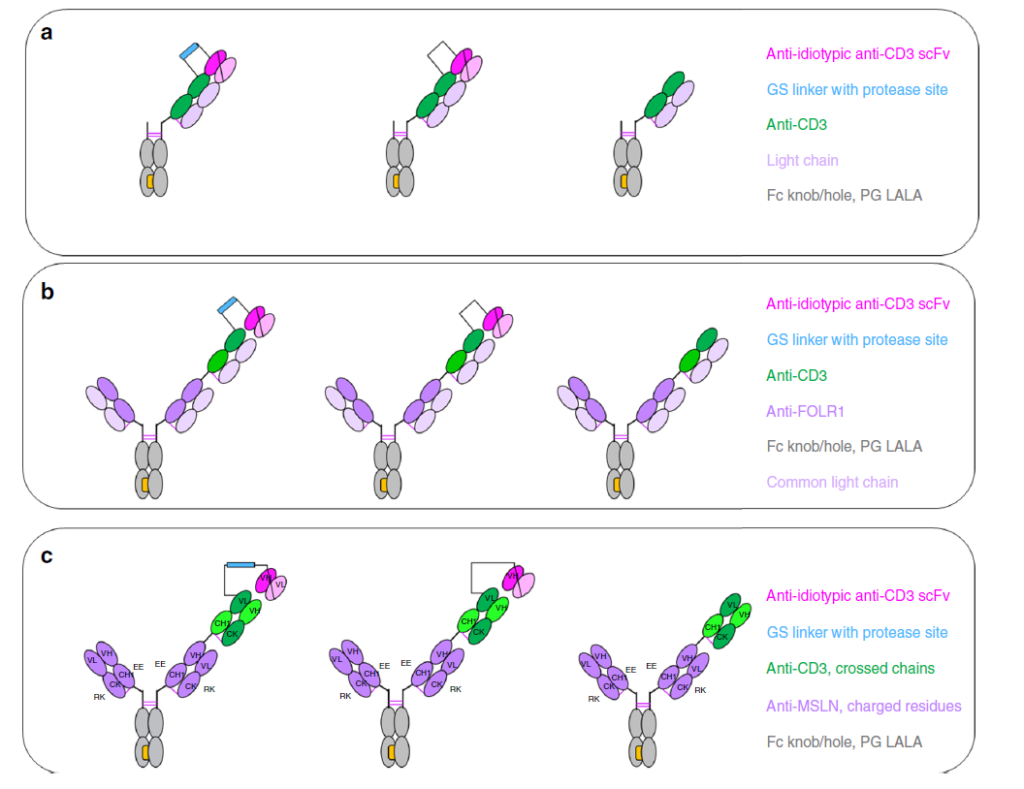
Figure 2 Enzyme-specific activation of CD3-IgG and TCB antibody design
Binding of activated Prot-TCB to CD3 antigen
In order to verify whether the digested Prot-TCB can effectively bind to the CD3 antigen, the author used an electron microscope to observe the structure of the antibody. The negative staining results showed that the activated antibody changed significantly after binding to the fused Fc CD3 antigen, which showed After the antibody is digested, it does not affect the binding of the antibody to the CD3 antigen
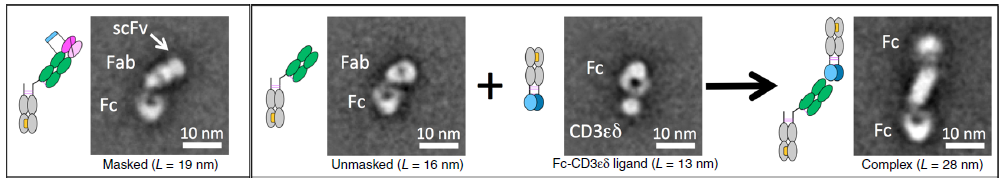
Figure 3 Negative staining electron microscopy to detect the activity of activated Prot-TCB antibody
The level of antigen expression determines the blocking efficiency of the Prot-TCB antibody to the CD3 binding site
In vitro experiments have shown that Prot-TCB kills tumor cells in a dose-dependent manner (Figure 4a-4f), while TCB using non-cleavable linkers will have weaker effects at higher concentrations of antibodies, or no effect.
The killing effect of cells with different antigen concentrations and the test results of Granzyme B prove that the inhibitory effect of Prot-TCB (Prot-FOLR-TCB, MMP-matA site) on tumor cells is related to the antigen level. When the antigen level is high, Prot-TCB (Prot-FOLR-TCB, MMP-matA site) The effect of FOLR-TCB and MMP-matA site is similar to that of FOLR-TCB; when the antigen level is low, Prot-FOLR-TCB and MMP-matA site have weaker dose-dependent cytotoxicity and granzyme B release.
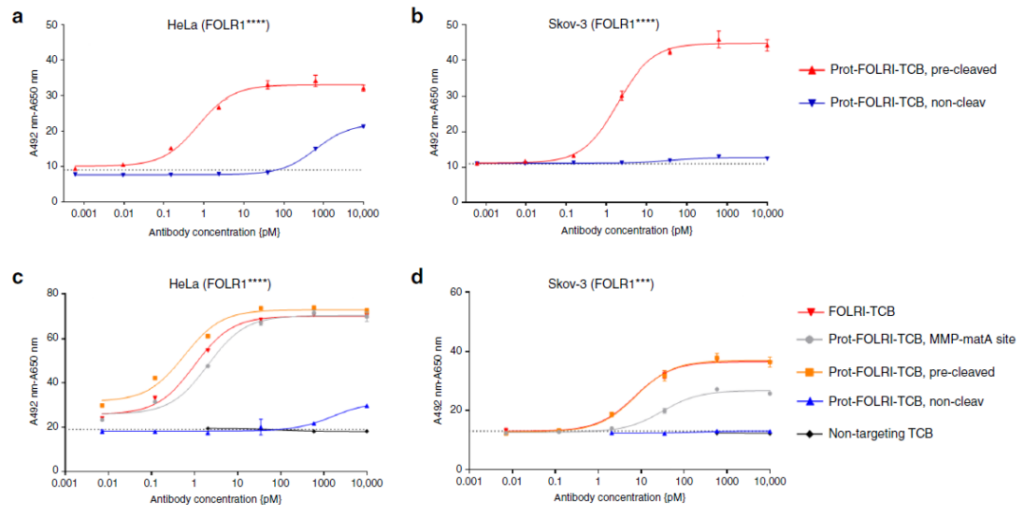
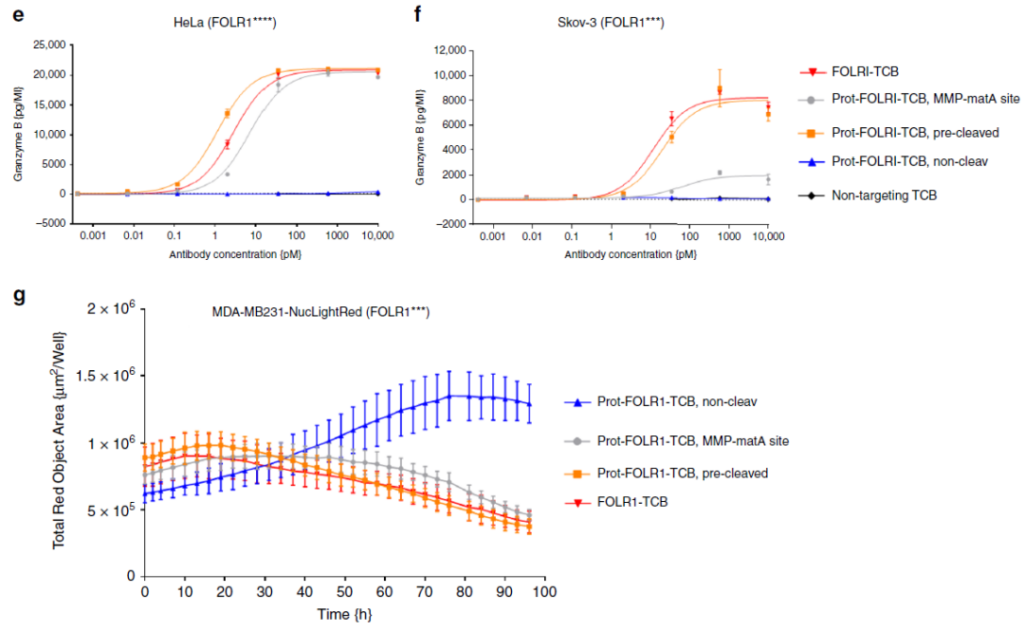
Figure 4 The efficacy of Prot-FOLR1-TCB in detecting cancer cells with different antigen densities
Activation of Prot-FOLR1-TCB by tumor tissue
Tumor-derived tissues were directly incubated with Prot-FOLR1-TCB containing different restriction sites without digestion to detect the activation of T cells.
The results showed that in normal tissues (normal tissues containing the FOLR1 target), Prot-FOLR1-TCB at any restriction site did not activate T cells, but FOLR1-TCB could activate T cells (Figure 5a); in tumors In tissues, Prot-FOLR1-TCB with different restriction sites has different activation efficiency, and some are even higher than FOLR1-TCB (Figure 5b, 5c).
These results prove that Prot-FOLR1-TCB can be specifically activated in tumor tissues, but different restriction sites have different activation efficiencies.
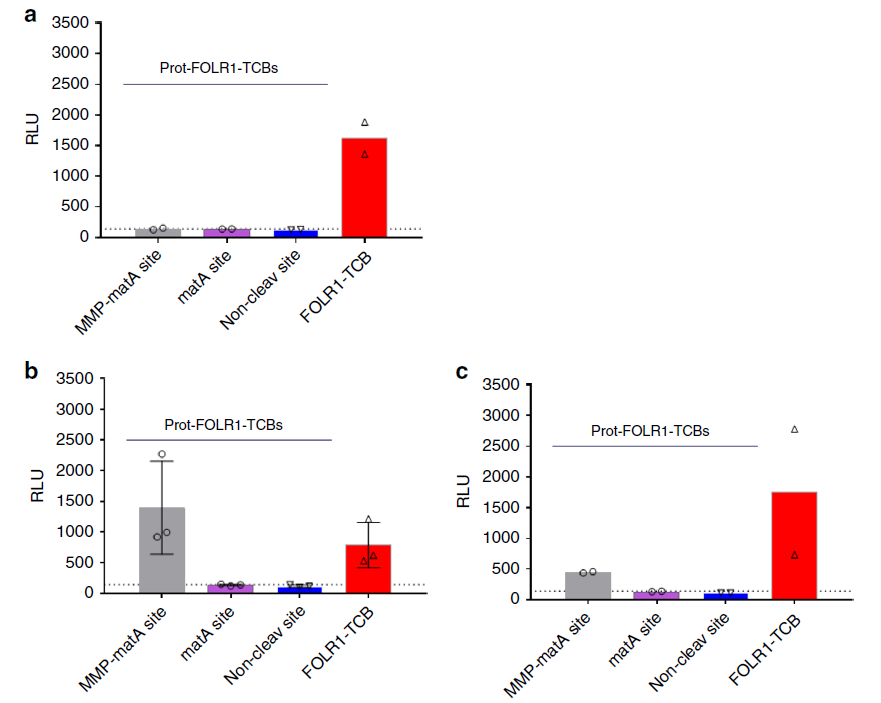
Figure 5 Prot-FOLR1-TCB can be specifically activated by patient-derived ovarian cancer tissue
Stability of Prot-FOLR1-TCB in blood
The stability of Prot-FOLR1-TCB is verified by intravenous injection of Prot-FOLR1-TCB containing different restriction sites. For example, healthy mice are used to verify the stability of Prot-FOLR1-TCB. Seven days after the antibody is injected, there are approximately 35 antibodies containing the restriction site of MMP-matA site. % Is activated, while only about 5% of MatB and matC containing a single restriction site are activated, which indicates that antibodies containing a single restriction site MatB or matC are more stable.
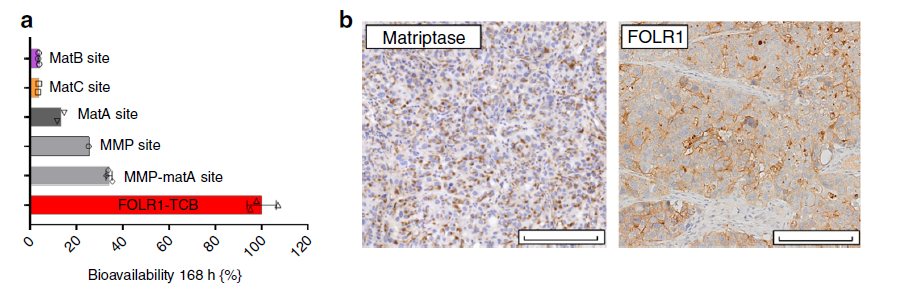
Figure 6 Stability of Prot-FOLR1-TCB with different restriction sites
PDX BC004 animal model verifies the effectiveness of Prot-FOLR1-TCB
Patient-derived tissue animal model experiments show that Prot-FOLR1-TCB containing matB restriction sites has a better tumor-inhibiting effect, even comparable to FOLR1-TCB.
The Prot-FOLR1-TCB with non-cleavable linker has the same pharmacological effect as the solvent and has almost no tumor-inhibiting effect. Among the animals treated with Prot-FOLR1-TCB containing matB restriction sites, the number of CD3-positive T cells was the largest, even better than that in the FOLR1-TCB treatment group.
These results prove that Prot-FOLR1-TCB can specifically activate T cells in tumors and inhibit tumor growth.
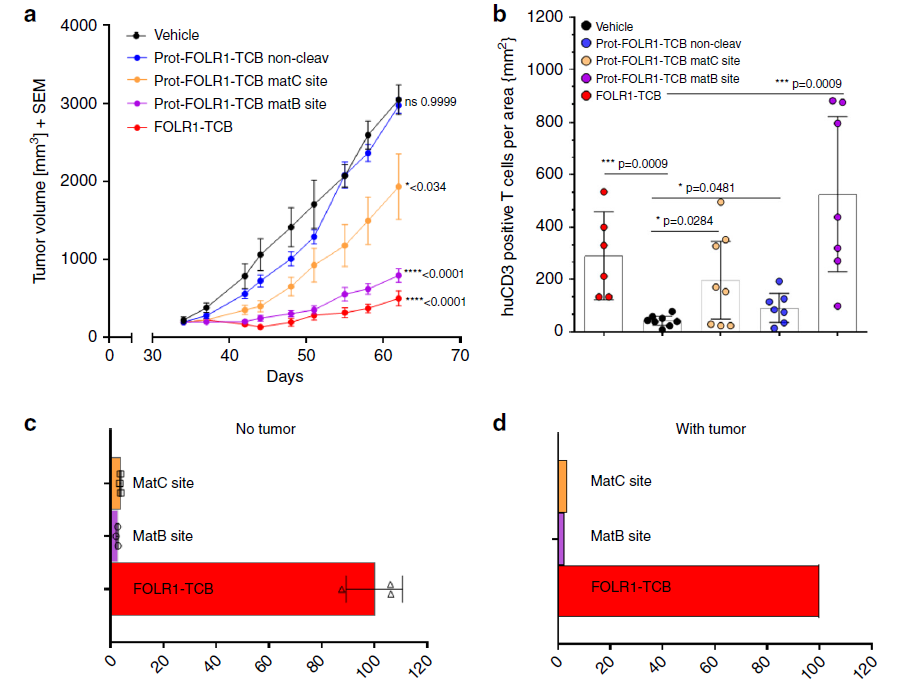
Figure 7 The efficacy of Prot-FOLR1-TCB in PDX BC004 animal model
Sum up
The TCB bispecific antibody has a good therapeutic effect in hematoma, but it is not satisfactory in solid tumors. This is mainly because most of the targets in solid tumors are also expressed in normal tissues, thus forming an On-target off-Tumor effect. .
The Prot-TCB bispecific antibody platform invented by Roche uses enzymes expressed in tumors to specifically activate antibodies in the tumor microenvironment. In tissues, the antibodies cannot be activated, thus avoiding the on-target off-target of antibodies. Tumor effect.
At present, there are many platforms that use enzyme-specific activation antibodies specifically expressed in tumors, such as Probod, SAFEbody, etc., and there are also technologies that use low pH activation antibodies in the tumor microenvironment, such as CAB. The application of these technologies can increase the dosage of antibodies, thereby expanding the therapeutic window, so there will be better therapeutic prospects.
(source:internet, reference only)
Disclaimer of medicaltrend.org



