Analysis of RCL/RCR in vector-transduced T cell products
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Analysis of RCL/RCR in vector-transduced T cell products
Analysis of RCL/RCR in vector-transduced T cell products. With the introduction of viral vectors into CAR-T products, researchers must prove its effectiveness and safety [1-4].
At present, the γ-retroviral vectors and lentiviral vectors used in the preparation of CAR-T cells are replication-defective vectors. Although the risk of replication-competent lentivirus (RCL) is theoretically low, it must obtain safety requirements. , This hypothesis must be strictly tested.
There are still potential risks of producing RCR or RCL during the preparation method and preparation process of viral vectors. For example, due to the shuttle vector, packaging vector and endogenous reverse transcription elements in packaging cells during the production process of viral vector, or between viral vector and T Homologous or non-homologous recombination occurs between the endogenous reverse transcription elements of the cell, resulting in the production of RCR or RCL.
For example, recombination has been demonstrated in the early retroviral packaging cell lines used to produce gamma retroviral vectors [6]. The results have shown that replication-competent retroviruses cause malignant tumors in murine and primate models of gene therapy [7, 8].
For example, although early studies have shown that murine γ-retrovirus MLV does not cause serious safety problems in non-human primates [12-13], the researchers conducted a study on severe immunodeficiency rhesus monkeys. In the experiment of in vitro infusion of bone marrow progenitor cells, it was found that out of 10 animals infused with bone marrow progenitor cells transduced by γ-retroviral vector, 3 animals developed lymphoma and died within 200 days [14], The cause analysis results showed that RCR can be detected in the lymphoma tissues of animals, and γ-retroviremia caused by RCR can be detected in infected animals [15], thus confirming the true culprit of lymphoma in animals It is RCR, which is caused by the contamination of RCR with the γ-retroviral vector that transduces bone marrow progenitor cells.
The risk of RCR/RCL to patients is manifested in two aspects: On the one hand, they can be integrated into the cell genome like retroviral vectors, resulting in activation of proto-oncogenes, destruction of tumor suppressor genes or promoting cell growth due to integration. The increased expression of the factor of the virus causes the risk of secondary tumors; on the other hand, because of its replication ability, it can produce a virus with the ability to replicate. In addition, the use of membrane proteins that can infect a variety of cells greatly increases integration and causes secondary tumors. The risk of secondary tumors.
The US FDA requires that for γ-retroviral vectors and lentiviral vectors, RCR/RCL testing needs to be carried out throughout the production process and at different stages to ensure that there is no RCR/RCL contamination [6-7]. For the control of RCR, the main cell bank for vector production, working cell bank, production terminal cells, vector supernatant and vector-transduced cells cultured in vitro for more than 4 days need to be tested.
For the control of RCL, the RCL test points mainly include terminal cells that produce viral vectors, vector supernatants, and vector-transduced cells. In addition, in clinical studies, for patients receiving vector-transduced cell infusion, patient samples should be tested for RCR/RCL. It is slightly different from the FDA’s view of detecting vector supernatants. The European Pharmacopoeia requires RCR/ RCL detection of purified viral vectors.
The methods for detecting RCR include two types of cell infectivity tests, namely S+/L- test and label rescue test [35]. In these experiments, there must be an expansion stage, that is, the sample to be tested and the sensitive cells are co-cultured for at least 3 weeks, the possible RCR is amplified, and then the amplified sample is inoculated on the indicator cells to be tested. The expression of lesions or specific products indicates the presence of RCR.
As for the RCL detection method, the FDA recommends the use of sensitive cells to culture and amplify the RCL that may be present in the viral vector, and to detect at the end of the culture [7]. Since the human T cell line C8166 is highly sensitive to HIV and lentiviral vectors enveloped by VSV-G, it is mostly used for RCL expansion culture and detection [41-43].
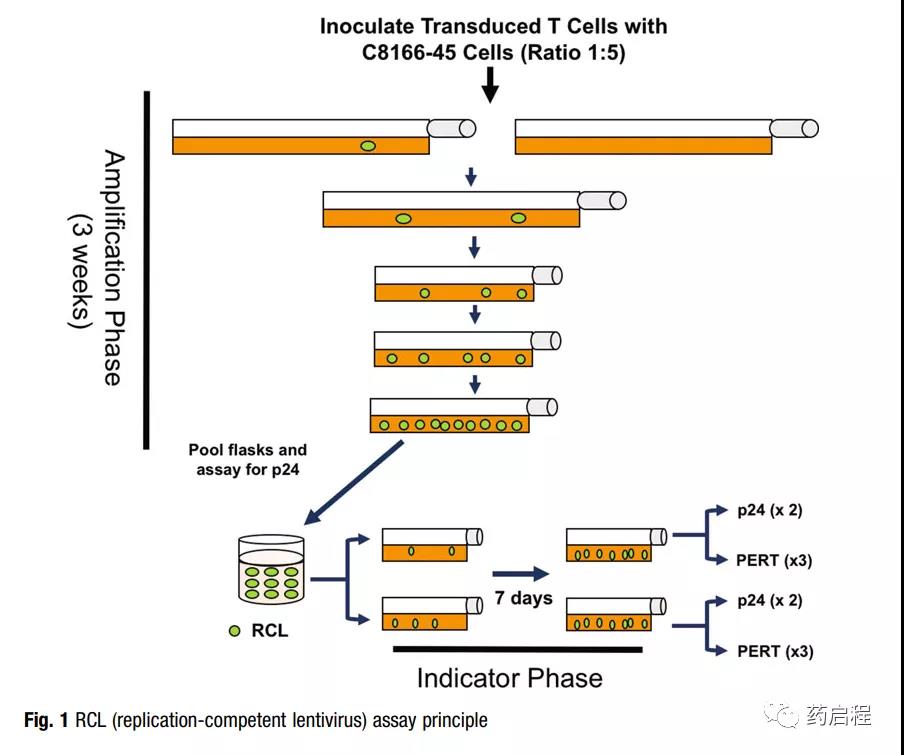
The authors used biological-based analysis methods to evaluate carrier products [9, 10] and transduced T cell products for cancer immunotherapy trials [11]. The test is designed to meet the latest recommendations of the U.S. Food and Drug Administration (FDA) [12].
RCL analysis involves an amplification stage (5 passages within 3 weeks) to allow virus amplification (Figure 1). The extended incubation period is designed to allow any slow-growing virus to reach a level sufficient for detection.
The virus was amplified on the T cell line C8166-45, which has been highly infected with VSV-G pseu modified vector particles and can amplify HIV-1 to high titer [10, 13].
After the 3-week expansion phase, the culture medium in the fusion culture medium is filtered and added to the original C8166-45 cell culture medium (indicator phase medium).
After one week of passage, two methods were used to test the RCL of the medium in the indicator phase culture: p24 ELISA for detecting viral capsids and product enhanced reverse transcriptase (PERT) analysis [14]. Although RCL is expected to have these two components, the recombinant virus may also be a hybrid between the vector packaging sequence and the human endogenous retroviral sequence.
In theory, including these two detection methods will increase the detection of mixed RCL.
(source:internet, reference only)
Disclaimer of medicaltrend.org



