What’s the New concept of vaccine adjuvant science ?
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
What’s the New concept of vaccine adjuvant science ?
What’s the New concept of vaccine adjuvant science ? Adjuvants are an integral part of the vaccine, which can enhance the breadth, breadth and persistent immune response of the vaccine.
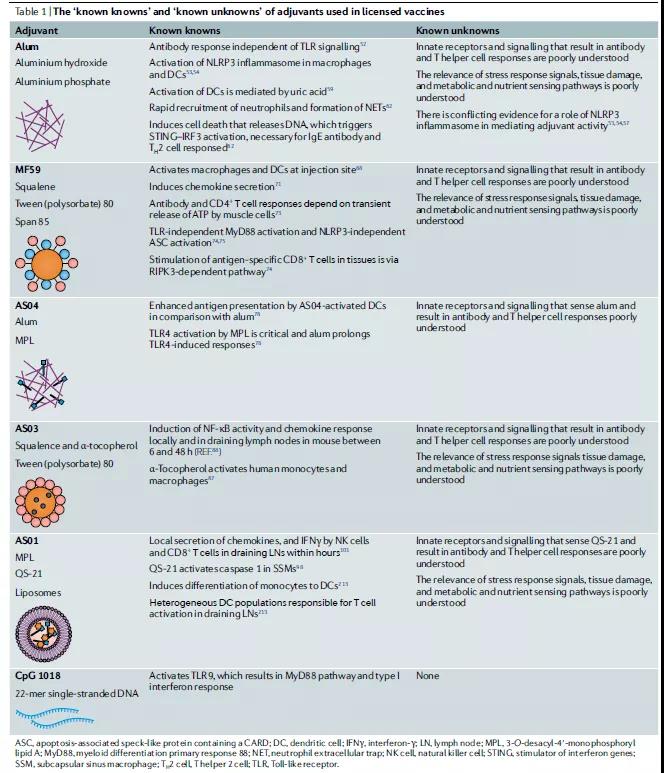
Since the 1990s, in addition to alum, scientists have discovered five adjuvants that can be used in vaccines, but the molecular mechanism of these adjuvants is only partially understood.
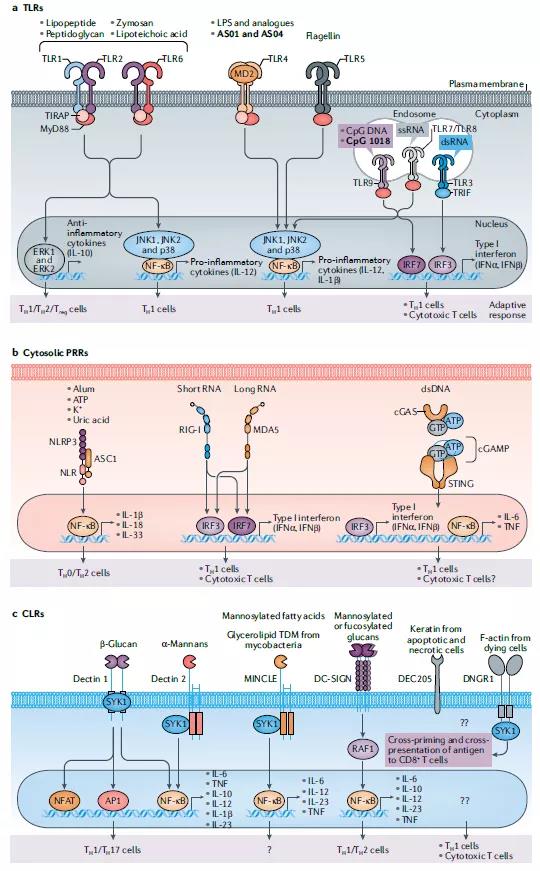
The discovery and research of pattern recognition receptors (PRRs) has brought new ideas for the development of new adjuvants, especially molecular adjuvants.
PRRs are a type of recognition molecules that are expressed in innate immune cells and can recognize pathogen-associated molecular patterns (PAMPs) PAMPs.
PRRs mainly include Toll-like receptors (TLRs), NOD-like receptors (NLRs), RIG-I-like receptors (RLRs), scavenger receptors (SRs), mannose receptors (MRs) and myeloid cell trigger receptors. Body (TREMs) and so on.
Recently, the development of many molecular adjuvants has benefited from the advancement of PRRs research. Molecular adjuvants refer to various immune-related molecules that can non-specifically change or enhance the body’s specific immune response to antigens and molecules that have immunostimulatory effects.
In this article, we review “known knowledge” and “known unknown” adjuvants, discuss these emerging concepts, and emphasize that as the knowledge of innate immunity and systemic vaccinology continues to expand, we are revitalizing their use for the prevention of COVID-19 And the science and development of new adjuvants for future pandemic vaccines.
Adjuvants in licensed vaccines
Although only a few adjuvants are available for clinical use, it is now clear that many vaccines that have been safely used in billions of people contain endogenous adjuvants. The description of innate immunity and key natural receptors, PRRs, has made significant conceptual progress, making people realize that many widely used live vaccines partly induce immune responses by activating specific PRRs23. These vaccines are composed of live attenuated pathogens that activate innate immunity by expressing various PAMPs. For example, the yellow fever attenuated live vaccine (YF-17D) is one of the most effective vaccines developed and vaccinated to 600 million people worldwide. -I and melanoma differentiation-associated protein 5 (MDA5) (Figure 1).
It is important that the TLR signal is on the immunogenicity of YF-17D, but the additional impact of sustained antigen expression during the replication of the attenuated virus should not be underestimated. Similarly, the BCG-Gelling (BCG) tuberculosis vaccine, of which more than 4 billion doses have been vaccinated, activates TLR2, TLR4, TLR9 and CD209 antigens (also known as DC-SIGN). In addition, certain inactivated vaccines can also trigger TLRs, such as seasonal influenza vaccines.
These findings further point to the continued and reasonable development of TLR agonists as key targets for adjuvant design, especially in subunit vaccines that do not contain endogenous adjuvants. Many studies have demonstrated the effectiveness of synthetic TLR ligands as vaccine adjuvants in mice and non-human primates (NHPs), and promote enhancement and enhancement when vaccination with recombinant protein antigens, virus-like particles or DNA-encoded antigens. A longer-lasting antibody response.
In fact, live virus vaccines can usually induce a strong and long-lasting antibody response in humans, which increases the possibility that inactivated vaccine injection adjuvants can also induce such a strong and long-lasting antibody response. Based on the discovery that the yellow fever vaccine binds to a variety of DC subgroups through multiple TLR ligands 24, we designed synthetic nanoparticles containing TLR4 and TLR7/TLR8 ligands as adjuvants for use with soluble protein antigens ( Such as chicken ovalbumin or influenza hemagglutinin) used in combination.
Recent studies have shown that sustained-release drug delivery systems that release antigens with delayed kinetics (such as osmotic pumps) can improve the magnitude, quality and durability of the antibody response. Although this is due to the longer retention time of the antigen in the lymph nodes and the enhanced GC response, the effect of adjuvants on the continuous activation of natural cells has not been fully evaluated. In this case, we and others discovered that 3M-052, a new TLR7/TLR8 agonist, is slowly released from the site of administration, leading to the activation of monocytes and DC for 3-4 weeks, and the induction continues The magnitude of the antibody response and LLPC was much larger than that observed by alum (up to 100 times) and lasted until the study was terminated 70 weeks after immunization.
Alum
Alum is the most commonly used adjuvant and is used in humans to induce antibody responses and CD4+T helper cell responses (Table 1). These helper T cell responses are the bias of helper T cell 2 (TH2) in mice, but this bias is not clear in humans. For a long time, people have believed that alum mainly mediates its adjuvant effect through the “warehouse effect” mechanism, which involves the slow release of antigens from immune sites. Alum actually has several action systems on the immune system.
As mentioned above, although TLR seems to be critical to the immunogenicity of live virus vaccines, such as the yellow fever vaccine YF-17D immunostimulated immune response, alum plus antigen does not affect the key to participating in TLR signaling in mice lacking this antibody. Adaptor protein: MyD88 or TIR domain contains adaptor interferon beta (TRIF). This indicates that the effect of adjuvants is to dissolve alum through a signal independent of TLR. Subsequent studies have shown that alum activates the NLRP3 inflammasome, although the relative importance of mediating the effects of alum adjuvant is contradictory.
Alum can also induce uric acid-mediated inflammatory DCs activation by causing tissue damage, thereby enhancing adaptive immunity. For example, in mice, the addition of antigen to alum induces immune cell death and subsequent release of host cell DNA, which stimulates an antigen-specific IgE response to the TH2 cell response. TH2 cells respond with a pathway that relies on tank-binding kinase 1 (TBK1) and transcription factor IRF3, which are key components of the signal, mediated by the cytosolic DNA sensor STING62 (Table 1).
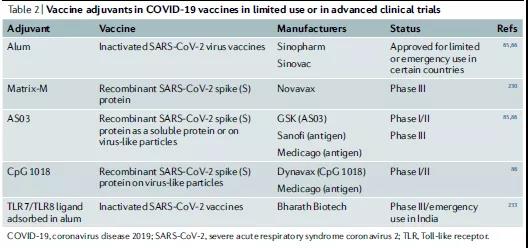
Alum is also used to inactivate the virus COVID-19 vaccine, and some countries have been approved for limited or emergency use (Table 2). Therefore, the mechanism of action of alum is complicated. In small animals, several factors may work at the same time, and the role of the formula cannot be ignored.
MF59 adjuvant
The research of MF59 adjuvant began in the late 1980s. Under the background of recombinant DNA at that time, many new protein antigens can be used for vaccine preparation.
Take squalene as an example, which is an oil extracted from shark liver and a key component of the adjuvant MF59, which has been used in seasonal influenza vaccines. MF59 is thought to act by stimulating nearby cells to secrete chemokines (signal transduction) to encourage other cells to produce more chemokines. This chain reaction can eventually attract immune cells to help the body recognize pathogens.
AS series adjuvant
AS (adjuvant System, AS) adjuvant is a series of human vaccine adjuvants developed by Glaxo Smith Kline (GSK), divided into AS01, AS02, AS03 and AS04. Currently, there are 7 new adjuvants Approved to be used in human vaccines are V Irosomes MF59, Imiquimod, Resiquimod, AS01, AS03 and AS04. The emergence of these new adjuvants broke the “outstanding” situation of aluminum adjuvants in human vaccine adjuvants.
Although it has been established that adjuvants can enhance human antibody responses to vaccination, to date, it has not been shown to the extent that adjuvants can induce antigen-specific CD8 + T cell responses stimulated by live virus vaccines (such as YF-17D). Some people believe that the initial dose and persistence of the antigen are the main determinants of CD8 + T cell response. However, the analysis of the immune response induced by the human live virus vaccine shows that both the antigen load and the adjuvant signal play a key role in inducing the adaptive immune response.
Cytosine phosphate guanosine 1018
TLR9 has three types of CpG oligonucleotide ligands, which can be distinguished by different nucleotide sequence motifs and their ability to stimulate IFNα in plasma cell DCs103 (Table 1). The TLR9 agonist CpG 1018 is a 22-mer non-methylated CpG-B oligonucleotide and an effective TH1 cell adjuvant, which can stimulate the strong activation of B cells and NK cells. CpG1018 is currently being evaluated as a potential vaccine adjuvant for the COVID-19 vaccine in clinical trials (Table 2).
Adjuvant and CD8+ T cells
In mice, several immunogens combined with a series of adjuvants can induce effective CD8 + T cell responses. In contrast, in humans, only live viral vaccines, such as yellow fever and smallpox, can induce very high antigen-specific effect CD8 + T cell responses and memory CD8 + T cell responses. Because these are replicative vaccines, these studies highlight that the initial viral load (antigen) determines the strength of the human CD8 + T cell response.
Experiments conducted in YF-17D mice showed that the activation of DC drives the induction of CD8 + T cells through multiple TLRs and the resulting MyD88 signal. In addition, YF-17D sends out signals through RNA sensors RIG-1 and MDA5. Therefore, an important question that needs to be resolved is whether there is an adjuvant that can mimic the efficacy of live vaccines in inducing human CD8 + T cell responses. Experience has shown that even when delivered with a strong adjuvant, the subunit vaccine will not induce a human CD8+ T cell response, at least it can be measured in the blood.
This is not just a problem of DCs
Dendritic cells have always been considered as the main cellular target vaccine adjuvant. In fact, there is clear evidence that dendritic cells are necessary adjuvant active TLR ligands. Therefore, in mice lacking dendritic cells, the adjuvant activity of TLR ligands is severely impaired. Even for non-TLR-based adjuvants such as alum or MF59, the conditional absence of DC or DC subgroups can cause severe damage to the adaptive immune response. These studies have clearly used dendritic cells as a sensitive adjuvant and one of the key cell types that regulate adaptive immunity.
However, the immune system is a complex network of interacting cell types. Although dendritic cells play a central role in innately sensing and coordinating immune responses, new evidence highlights the importance of other cell types in this process. Key role. For example, as mentioned earlier, the administration of alum and MF59 induced transcriptional changes in muscle cells that were greater than in dendritic cells. The release of ATP from muscle cells induced by MF59 is critical to its adjuvant activity. Future work should focus on a more comprehensive assessment of the natural sensations of different types of cells participating in adjuvants and their mechanisms for coordinating adaptive immune responses.
It’s not just a TLR problem
Although the main focus of adjuvant discovery in the past decade has been to target the TLR pathway, it is now clear that other PRRs can be targeted to achieve adjuvant effects. The receptors that may be developed for a new generation of adjuvants include NLRs, RIG-I-like receptors (RLRs), CLRs and STING ligands. It is worth noting that the signals sent by most PRRs (including TLRs) can cause local tissue and cell damage, and the damp released from this seems to be a key component of the activity of several adjuvants. For example, the cytoplasmic sensor NLRs of bacterial PAMPs recognize a variety of cell products, including ATP, uric acid, and K+ efflux, indicating that this activation may be mediated by cell damage.
Chen et al. recently discovered that circular RNA activates RIG-I in vivo and can be used as an adjuvant to induce cell-mediated and antibody responses. When the expressed antigen is effectively presented by the major histocompatibility complex (MHC) class I in the cell, it will be interesting to further evaluate whether the circular RNA-encoded protein can induce a stronger and more effective CD8+ T cell response. problem.
Metabolism, cell death and epigenetics
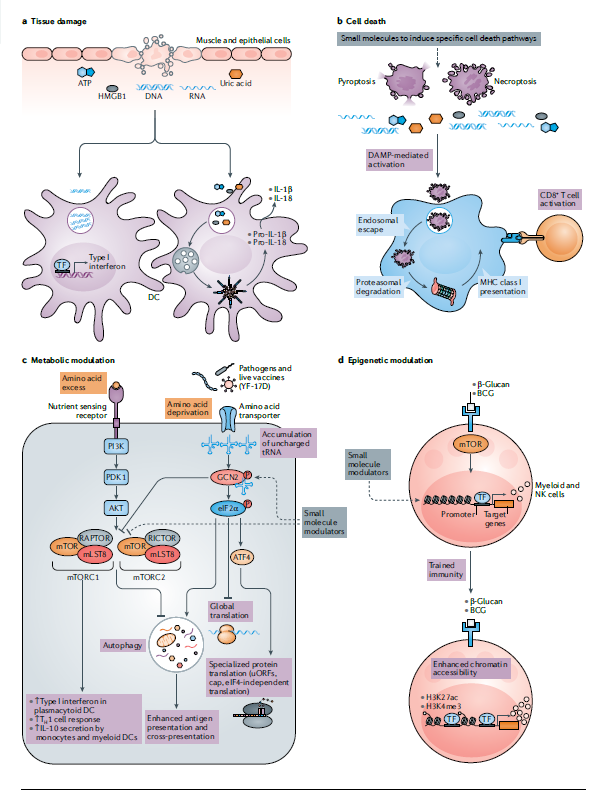
In the past decade, it has become increasingly clear that the innate immune system can not only sense microorganisms through PRRs, but also sense various tissue damage and stress signals. For example, tissue damage can be caused by trauma, autoimmunity or infection, and lead to cell death and the release of excessive moisture outside the cell, such as ATP or uric acid, or DNA or RNA fragments or high mobility group box 1 (HMGB1), which can Activate DC to stimulate adaptive immunity. This raises the question whether these natural activation pathways can be used in the design of new adjuvants. In fact, there is new evidence that the adjuvants that have been used clinically can indeed stimulate the immune response in this way (Figure 2a).
Cell death adjuvant
Cell death has also become a key regulator of immune response. Early research by Bevan and colleagues showed that exogenous antigens that normally cannot enter the cytoplasm of DCs can be transferred to the cytoplasm, where they can be processed or displayed on MHC class I cells to stimulate CD8+ T cell immunity. The follow-up work of many laboratories helped to establish the way of DC antigen presentation, and proved that DC can also obtain antigens from apoptotic cells and cross-present these antigens to CD8+ T cells. However, research in the past decade has revealed many ways of cell death, and many works have focused on understanding the mechanism of necrosis. Interestingly, RIPK3-mediated stimulation of CD8+ T cells occurs through a mechanism independent of MLKL, suggesting that RIPK3-mediated CD8 activation + T cells through a non-necrotic ptosis mechanism (Figure 2b)
Metabolic adjuvants
Another concept that has developed strongly in recent years is that the metabolic state of bone marrow cells (such as macrophages and dendritic cells) can plan their inherent responses and the ability to stimulate T cells. Therefore, dcs in different stages of maturity or in different tissues have different metabolic states. For example, the central metabolic regulator mTOR complex plays a major role in mediating TLR-induced plasmacytoid DC secretion of type I interferon, affecting IL-12 secretion in myeloid DC, and metabolically reprogramming lung DC to make Allergic inflammation shifts from eosinophilic TH2 to neutrophilic TH17 cell polarity through a mechanism that leads to increased oxidation of IL-23 and fatty acids.
Epigenetic adjuvant
Another concept that has recently emerged is innate immune memory or “training immunity.” It has been suggested that bone marrow cells, such as monocytes and macrophages 163 or NK cells, acquire memory-like characteristics after being stimulated by PAMPs. This “innate memory” relies on continuous epigenetic programming, which is caused by primary stimuli.
After secondary stimuli, cells either overreact or underreact. Vaccines (such as BCG) and PAMP (such as β-glucan) induce trimethylation of histone H3 at Lys4 (H3K4me3) and acetylation of H3 at Lys27 (H3K27ac) in monocytes and macrophages (Figure) 2d). We can imagine that small molecules that target monocytes or other myeloid cells undergo epigenetic reprogramming to stimulate the high activation of specific aspects of the innate response, such as an antiviral state that can enhance the resistance to viruses.
Over a period of time, maybe a few weeks or so. Such epigenetic adjuvants can be used to imprint an enhanced antiviral status and confer resistance to viruses that are widespread in humans. Among susceptible populations, during pandemics such as COVID-19, it is particularly advantageous to use this epigenetic adjuvant to enhance antiviral resistance in a limited time that may be several weeks.
Efficacy of adjuvant formulation
Synthetic molecules and natural products.
Although the most successful adjuvants, including those in licensed products, are mainly composed of ingredients from natural sources, we do not believe that this necessarily reflects the best way to “success” in the early 21st century. It must be admitted that the ingredients used in the most successful adjuvants were generally available in the early 1990s and before.
They are the best materials because they are readily available and effective. However, people are now increasingly aware of the key role of innate immunity to adjuvants. Synthetic chemistry has promoted the development of new molecules that can be better designed as pure agonists.
Although the initial plan focused on the discovery of TLR agonists, the search for new agonists that naturally activate the system soon followed. The inherent properties of these newly discovered adjuvants make them more suitable for product development than the original natural molecules they may replace.
Formulating and creating new formula methods
Since this small molecule agonist benefits greatly from the presentation of microparticles, chemical manipulation can make it suitable for formulation into a preferred delivery system, with flexibility in solubility and compatibility. Recent studies on animal models have emphasized the value of nanoparticle vaccines
Several formulation methods have been established for adjuvants in licensed products. These include insoluble aluminum salts used as adsorbents, low oil content for easy injection, oil-in-water emulsions, and liposome delivery systems.
Include only the necessary things.
Ensure that each component is necessary and add clear value to make it possible to improve the reasonable construction of adjuvants. AS01 is the most successful adjuvant among currently licensed products. Based on the efficacy level of this product, its efficacy on varicella-zoster is >97%.
New frame: Systemic vaccinology
The established method of developing vaccines containing new adjuvants has been described as one of the slowest processes in medicine. For decades, the development of adjuvants has relied on the systematic testing of drug candidates. Mouse molecular research has progressed into NHP models and eventually tested in humans (Figure 4a).
However, among all adjuvants that have shown great immunogenicity and effectiveness in animal models, only a few have proven to be safe and effective in humans. One of the main reasons for the lack of translation from mice to humans is their evolutionary differentiation 62 million years ago. Although their immune systems have extensive similarities (both T lymphocytes and B lymphocytes in both mice and humans), This produces important immune differences (such as differences in TLR7 expression in DC subgroups).
These main differences emphasize the necessity of using human models in adjuvant trials. The latest advances in systemic vaccinology have changed our ability to detect the immune response of human vaccines to an unprecedented level of accuracy.
For decades, vaccine manufacturers have relied on a single measurement method, usually serum antibody titers, to assess the immune response to vaccination. A major limitation of this method is that it cannot capture the complexity of the vaccine immune response, so it may not be possible to determine the relevant factors and key mechanisms of protective immunity.
Systemic vaccinology uses high-throughput technologies such as transcriptomics, metabolomics, high-dimensional cytometry, and epigenomics to comprehensively analyze the vaccine immune response, and use the data generated from these analyses to describe the relevant factors and mechanisms of vaccine immunity. In an independent study, Gaucher et al. found similar characteristics induced by yellow fever vaccines, which are associated with subsequent adaptive immune responses.
After these studies, some groups used this method to study the immune response to several other diseases, including influenza, malaria, meningococcal and pneumococcal infections, and varicella-zoster virus. Importantly, systematic vaccinology methods have been used to determine common vaccine induction characteristics in multiple flu seasons and in different populations (elderly, diabetics). These studies have identified characteristics related to the durability of the antibody response.
Systematic vaccinology research has produced many new insights on the mechanism of vaccine response. For example, the expression of TLR5 is induced within a few days after vaccination and is closely related to the antibody response after a few weeks. Subsequent mouse experiments showed that TLR5-deficient mice had impaired antibody responses to seasonal influenza vaccination.
This may be because flagellin from the gut microbiota sends a signal through TLR5 and provides an adjuvant signal to enhance the antibody response. Therefore, mice vaccinated with broad-spectrum antibiotics or sterile mice have impaired antibody response to influenza vaccine.
On the basis of these studies, we conducted a study on humans to evaluate the impact of the microbiota on the immune response to seasonal influenza vaccines by administering broad-spectrum antibiotics to healthy people before and after the seasonal influenza vaccination. These studies reveal the power of systematic vaccinology methods: these studies reveal the power of systematic vaccinology methods: first, in determining the molecular predictors of human vaccine response; then, experimental verification in mouse models; finally, Test the insights of these mechanisms in a new human study.
Promote adjuvant development
A new adjuvant development framework may place more emphasis on testing many potential adjuvant concepts in drug small-scale clinical trials (phase 0/I). Therefore, new adjuvants can be quickly tested in small first-phase (phase 0) human trials and systematic vaccinology methods to gain insights into the mechanism. The results of a recent clinical trial showed that the COVID-19 vaccine and another saponin adjuvant Matrix-M1 have high neutralizing antibody titers, but there is a lack of detailed knowledge about molecular and cellular immunity.
The role of such saponin adjuvants The mechanism mediates their influence. In addition, the systematic vaccinology method can not only be used to determine the mechanism of infection and the role of adjuvants, but also include which formulas are effective, the potential mechanisms of adverse reactions shortly after vaccination, and the rational design of the optimal dosage form of the vaccine. The results obtained from the 0/I phase research will contribute to the formation of the mechanical hypothesis.
Regarding adjuvants that can be tested in animal models or in vitro human organoid cultures (Figure 4b). Although the main focus of adjuvant discovery in the past decade has been on the TLR pathway, it is now clear that other PRRs can also be targeted to achieve adjuvant effects. The available receptors for potential use of the new generation of adjuvants include NLR, RIG-I-like receptor (RLR), CLR and STING ligands.
It is worth noting that the signals sent by most PRRs (including TLRs) can induce some local tissue and cell damage, and the DAMP released from this seems to be a key component of the activity of several adjuvants. Here, we propose an improved new model for rational design and iterative testing of new adjuvants—an interdisciplinary approach based on systemic vaccinology to accelerate the discovery and development of clinical adjuvants (Figure 4). Based on our experience in managing new adjuvants to the commercial approval of vaccine products, we want to emphasize the value of early use of human studies.
The “omics” data generated from these studies can facilitate the formation of new hypotheses about the mechanism by which candidate adjuvants stimulate strong and durable antigen-specific T cell and B cell responses. Such hypotheses can be retested in animal models, and the subsequent mechanistic insights can be used to design new adjuvant concepts. In the early stages of the adjuvant development pipeline, this method of combining human models with the field of systemic vaccinology has the potential for transformation.
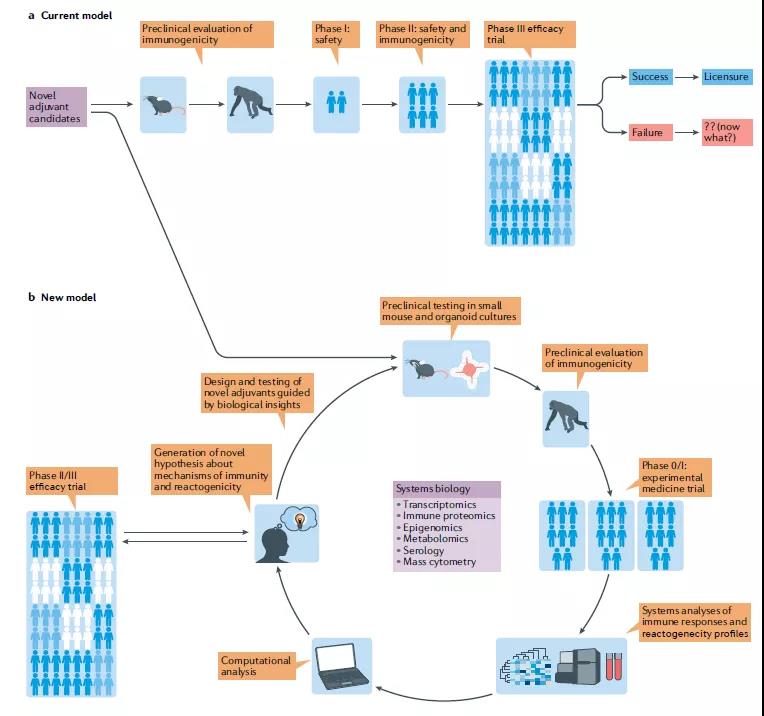
- The current model for developing vaccines containing novel adjuvants represents the process from systematic testing of new candidates in mice to the development of promising candidates to testing in non-human primates (NHPs) and The linear progression of final testing in humans at multiple stages of clinical trials.
- The new model proposed by the researchers relies on a process of repeated testing in mice, organ cultures, NHPs and humans. We recommend using small-scale experimental human trials as early as possible, and using systems biology methods to generate multi-parameter immune readings to generate new hypotheses and auxiliary concepts that can be retested in the preoperative model.
(source:internet, reference only)
Disclaimer of medicaltrend.org



