First TSLP-targeted monoclonal antibody: Tezepelumab Submit BLA
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
First TSLP-targeted monoclonal antibody: Tezepelumab Submit BLA
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- What is the difference between Atorvastatin and Rosuvastatin?
- How long can the patient live after heart stent surgery?
First TSLP-targeted monoclonal antibody: Tezepelumab Submit BLA. Blockbuster new medicine for asthma! AstraZeneca/Amgen’s first TSLP-targeted monoclonal antibody tezepelumab submit the application: it will set off a bloody storm!
Amgen recently announced that its partner AstraZeneca has submitted an antibody drug tezepelumab to the U.S. Food and Drug Administration (FDA) for the treatment of severe asthma Biological Licensing Application (BLA).
Tezepelumab is the first biological agent that can continue to significantly reduce the severity of the disease in a broad population of severe asthma patients. In 2018, the US FDA granted tezepelumab breakthrough drug designation (BTD).
Tezepelumab is a potential first-in-class drug that can block the action of thymic interstitial lymphopoietin (TSLP), which is an epithelial cytokine that plays a key role in asthma inflammation.
The drug BLA is based on the positive data of the PATHFINDER clinical program, including the positive results of the pivotal Phase 3 NAVIGATOR trial. The trial showed that in a wide range of patients with severe uncontrolled asthma, tezepelumab showed a statistically significant and clinically significant reduction in the annual exacerbation rate (AAER) of asthma compared with placebo.
It is worth mentioning that tezepelumab is the only biological agent that can consistently and significantly reduce the AAER in patients with widespread severe asthma, regardless of the patients’ baseline eosinophil counts in phase 2 and phase 3 clinical trials.
David M. Reese, MD, Executive Vice President of Amgen Research and Development, said: “Although there are currently available treatments for this complex and often debilitating disease, severe asthma is still uncontrollable for many patients.
The application brings us closer to providing a potentially transformative treatment option for a broad population of severe asthma patients, including patient populations with different phenotypes and different biomarkers.”
The NAVIGATOR trial is the first phase III trial that shows the therapeutic benefit of targeting TSLP for severe asthma. The results of the trial were announced in November 2020.
The data showed that the trial reached the primary endpoint: in the entire patient population, tezepelumab+SoC treatment increased the 52-week asthma exacerbation rate (AAER) compared with placebo + standard of care (SoC). ) A statistically significant and clinically significant decrease.
In this trial, the SoC was a medium-dose or high-dose inhaled corticosteroid (ICS) plus an additional control drug, with or without oral corticosteroids (OCS).
In addition, in the subgroup of patients with a baseline eosinophil count of <300 cells/μl, the trial also reached the primary endpoint: compared with placebo+SoC, tezepelumab+SoC treatment made AAER statistically significant and The reduction of clinical significance. Similar reductions in AAER were observed in the subgroup of patients with a baseline eosinophil count of <150 cells/μl.
In terms of safety, tezepelumab is well tolerated in patients with severe asthma. Preliminary analysis showed that there was no clinically significant difference in safety results between the tezepelumab treatment group and the placebo group.
The detailed data of the trial will be announced at the American Thoracic Society (ATS) 2021 International Conference to be held later in May 2021.
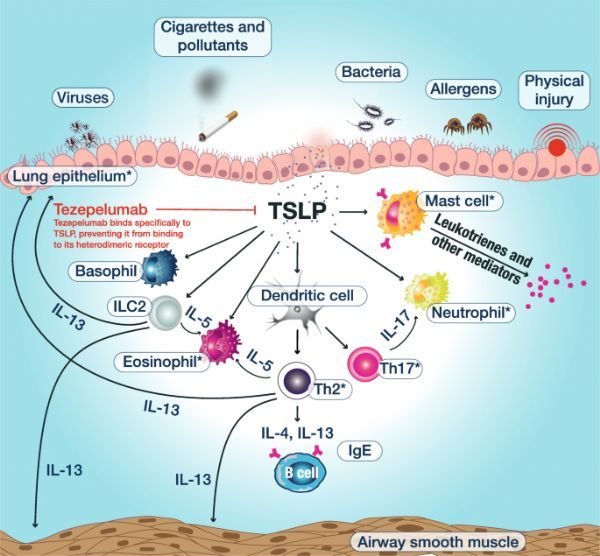
Mechanism of action of tezepelumab (picture from document PMID:33050900)
Tezepelumab: suitable for a wide range of severe asthma groups, will set off a bloody storm in the field of asthma
TSLP is an epithelial cytokine produced in response to pro-inflammatory stimuli (such as lung allergens, viruses and other pathogens), and plays a key role in the occurrence and persistence of airway inflammation. TSLP drives the release of downstream T2 cytokines, including IL-4, IL-5 and IL-13, leading to inflammation and asthma symptoms.
TSLP can also activate many types of cells involved in non-T2-driven inflammation. Therefore, the early upstream activity of TSLP in the inflammatory cascade has been identified as a potential target in a broad population of asthma patients.
Blocking TSLP can prevent immune cells from releasing pro-inflammatory cytokines, thereby preventing asthma from getting worse and improving asthma control.
Tezepelumab is a first-in-class anti-TSLP monoclonal antibody drug that specifically binds to human TSLP and blocks its interaction with the receptor complex, thereby preventing immune cells targeted by TSLP Release pro-inflammatory cytokines to prevent asthma attacks and improve asthma control.
Because of its role in the early upstream of the inflammatory cascade, tezepelumab may be suitable for a wide range of patients with severe uncontrolled asthma, regardless of the patient’s phenotype or T2 biomarker status.
Severe asthma is a debilitating disease that affects approximately 34 million people worldwide. Due to the complexity of severe asthma, many patients with severe asthma continue to experience symptoms and frequent worsening despite receiving standard care inhaled medications, currently available biological therapies, and oral corticosteroids (OCS).
The effect of tezepelumab is different from any other asthma biologics. Its target of action is a variety of inflammatory pathways, which can cause asthma symptoms and aggravation. Tezepelumab has the potential to change the care of the currently underserved population of severe asthma patients, including those who do not have an eosinophilic phenotype.
Currently, tezepelumab is being jointly developed by AstraZeneca and Amgen. The industry believes that if tezepelumab is successfully listed, it will set off a bloody storm in the field of asthma treatment, and its treatment population will be much larger than the currently marketed biological therapies, including GlaxoSmithKline (GSK) Nucala (mepolizumab, targeting IL-5) , Teva’s Cinqair (reslizumab, targeting IL-5), and currently developing biological therapies for the treatment of asthma, such as AstraZeneca’s own benralizumab (targeting IL-5 receptor alpha subunit [IL-5Rα]) and Sanofi’s Dupixent (targeting IL-4/IL-13), all four therapies only target specific inflammatory molecules that drive asthma inflammation, and are only suitable for certain types of severe asthma patients, that is, a subgroup of patients. Such as eosinophilic asthma.
First TSLP-targeted monoclonal antibody: Tezepelumab Submit BLA
(source:internet, reference only)
Disclaimer of medicaltrend.org



