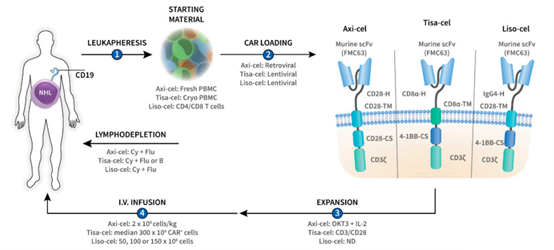
CAR structure and the purpose of each component
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
CAR structure and the purpose of each component
CAR structure and the purpose of each component. Today let’s talk about some knowledge about CAR structure and principles of CAR design.
CAR structure
CAR is a modular synthetic receptor composed of four main components:
(1) extracellular target antigen binding domain,
(2) hinge region,
(3) transmembrane domain,
(4) one or more intracellular Signaling domain.
Here, we will discuss the basic principles of CAR design.
Antigen binding domain
The antigen binding domain is the part of the CAR that confers specificity on the target antigen. Historically, the antigen-binding domain was derived from the variable heavy chain (VH) and light chain (VL) of monoclonal antibodies, which were connected by a flexible linker to form a single chain variable fragment (scFv). Classically, the scFv present in the CAR targets extracellular surface cancer antigens, leading to major histocompatibility complex (MHC)-dependent T cell activation, although MHC-dependent T cell receptors (TCR) have been used Simulate CAR to recognize intracellular tumor-associated antigens [7]. In addition to simply identifying and binding target epitopes, several features of scFv also affect CAR function. For example, the way of interaction between the VH and VL chains and the relative position of the complementarity determining region will affect the affinity and specificity of the CAR for its target epitope [8].
Affinity is a particularly important antigen-binding domain parameter because it fundamentally determines the function of CAR. In order to recognize antigens on tumor cells, induce CAR signal transduction and activate T cells, the antigen binding affinity of CARs must be high enough, but not high enough to cause activation and induce the death of CAR-expressing T cells and trigger toxicity [9,10].
Although affinity is undoubtedly one of the most important factors that further complicate matters, it has been proven that even scFv with similar affinity can differentially affect the function of CAR-T cells. Therefore, in order to optimize the binding of CAR to its target antigen, other factors must be considered, such as epitope location, target antigen density, and avoiding scFv related to ligand-independent tonic signaling.
Hinge area
The hinge or spacer is defined as the extracellular structural region that extends the binding unit from the transmembrane domain. The function of the hinge is to provide flexibility to overcome spatial barriers and contribute to the length to allow the antigen binding domain to enter the target epitope.
Importantly, the selected hinge seems to affect the function of CAR, because differences in the length and composition of the hinge region affect flexibility, CAR expression, signal transduction, epitope recognition, activation output intensity, and epitope recognition [11,12] . In addition to these effects, it has been proposed that the length of the spacer is critical to provide sufficient intercellular distance to allow the formation of immune synapses [13].
In principle, the “optimal” spacer length depends on the location of the target epitope and the level of steric hindrance on the target cell, where a long spacer can provide greater flexibility and allow more efficient access to the membrane proximal epitope or Complex glycosylated antigens, and short hinges can more successfully bind to epitopes at the distal end of the membrane [11,14,15,16]. However, in practice, the appropriate spacer length is usually determined empirically and must be tailored for each specific pair of antigen binding domains.
There are many examples in the literature. Short-spaced CAR (CD19 and carcinoembryonic antigen CEA) and long-spaced CAR (mucin 1 (MUC1)) are membrane near epitopes of receptor tyrosine kinase-like orphan receptor 1 (ROR1) [ 14]. The most commonly used hinge regions are derived from the amino acid sequence of CD8, CD28, IgG1 or IgG4. However, spacers derived from IgG can cause CAR-T cell depletion, thereby reducing their persistence in the body because they can interact with Fcγ receptors [17,18]. These effects can be avoided by selecting different spacer regions or by additionally designing the spacer regions based on functional or structural considerations.
Transmembrane domain
Among all the components of CAR, the transmembrane domain may be the least characteristic region. The main function of the transmembrane domain is to anchor the CAR on the T cell membrane, although there is evidence that the transmembrane domain may also be related to CAR-T cell function [19,20]. More specifically, studies have shown that the CAR transmembrane domain affects the expression level and stability of CAR, can play a role in signal transduction or synapse formation, and dimerizes with endogenous signaling molecules [19,20,21 ]. Most of the transmembrane domains are derived from natural proteins, including CD3ζ, CD4, CD8α or CD28. Since the transmembrane domain often changes according to the requirements of the extracellular compartment or intracellular signal transduction domain, the effect of one type of transmembrane and another type of transmembrane on the function of CAR has not been well studied.
It is worth noting that CD3ζ transmembrane can promote CAR-mediated T cell activation, because the CD3ζ transmembrane domain mediates CAR dimerization and incorporation of endogenous TCRs [19]. Compared with the CAR with the CD28 transmembrane domain, these beneficial effects of the CD3ζ transmembrane domain are at the cost of reducing the stability of the CAR. At the same time, the influence of the transmembrane domain and hinge region also seems to affect the cytokine production and activation-induced cell death (AICD) of CAR-T cells, because CAR-T cells with CD8α transmembrane and hinge domains release TNF And the number of IFNγ is reduced, and the sensitivity to AICD is reduced compared with CARs with these domains derived from CD2823.
Overall, studies have shown that by linking the proximal intracellular domains to the corresponding transmembrane domains, appropriate CAR-T cell signal transduction can be best promoted, while the commonly used CD8α or CD28 transmembrane domains are used Can enhance the expression and stability of CAR.
Intracellular signaling domain
It can be said that the most concerned focus in CAR engineering is to understand the effects of CAR costimulation, with the goal of generating CAR structures with the best internal domains.
The first generation CAR designed in the late 1990s contained the CD3ζ or FcRγ signaling domain [24]. Most CARs rely on CD3ζ-derived immunoreceptor tyrosine-based activation motifs to activate CAR-T cells [25]. However, only sending signals through these motifs cannot produce an effective T cell response [26]. The durability and durability of these first-generation CARs are not strong in vitro [26]. These findings are in line with clinical studies, which have shown limited or no efficacy [27,28]. Early in vivo models using B-cell malignancies demonstrated the importance of costimulation in the persistence of CD-19-targeted CAR-T cells [29]. By adding a costimulatory domain, the production and proliferation of IL-2 after repeated exposure to antigen can be improved [30].
(Picture from doi: 10.3390/pharmaceutics12020194)
After understanding the importance of costimulation for persistent CAR-T cell therapy, a second-generation CAR with a costimulatory domain in tandem with a CD3ζ intracellular signaling domain was produced [30,31].
The two most common costimulatory domains approved by the FDA, CD28 and 4-1BB (CD137), are associated with high patient response rates. The function and metabolic profile of the costimulatory domain are different. CARs with CD28 domain differentiate into effector memory T cells, mainly using aerobic glycolysis, while CARs with 4-1BB domain differentiate into central memory T cells and show mitochondrial biology Occurrence and oxidation increase. Metabolism [32].
Clinically, the second-generation CAR-T cells have produced a strong therapeutic response in several hematological malignancies, including chronic lymphocytic leukemia, B-cell acute lymphoblastic leukemia, diffuse large B-cell lymphoma and multiple bone marrow Tumors and the efficacy of second-generation CAR-T cells are currently being studied for solid tumors, including glioblastoma, advanced sarcoma, liver metastases, as well as mesothelioma, ovarian cancer and pancreatic cancer [33].
Despite its preclinical efficacy, several alternative costimulatory domains (such as inducible T cell costimulator (ICOS) [34], CD27 [35], MYD88 and CD40 [36] and OX40 (CD134) [37] have been Shows preclinical efficacy. Clinical research is still in progress. It is speculated that costimulation of only one domain will produce incomplete activation, leading to the generation of a third-generation CAR, which contains two costimulations in tandem with CD3ζ Structure domain []38.
Preclinical studies of the third-generation CAR have yielded different results. Specifically, compared with second-generation CARs, CARs that combined CD28 and 4-1BB signals resulted in stronger production of cytokines in lymphoma, and lung metastases showed improved anti-tumor responses in vivo. In leukemia and pancreatic cancer models, the third-generation CAR has not shown the advantages of in vivo treatment in their respective models, and cannot outperform the second-generation CAR [40,41].
(source:internet, reference only)
Disclaimer of medicaltrend.org



