Global cancer cell therapy research and development pipeline summary
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Nature: Global cancer cell therapy research and development pipeline summary, rapid growth, China and the United States lead
Nature: Global cancer cell therapy research and development pipeline summary. Cancer cell therapy belongs to immunotherapy. It is through the transformation of autologous or foreign immune cells in vitro and infused back into the patient’s body, thereby enhancing its killing effect on cancer cells, and then treating cancer. Current cancer cell therapy includes CAR-T, TCR-T, CAR-NK, TIL, CTL and so on.
In August 2017, the FDA approved Novartis’s CD19-targeting CAR-T cell therapy Kymriah for the first time. Since then, it has successively approved the marketing of three CD19-targeted CAR-T cell therapies.
In March of this year, Abecma, a CAR-T cell therapy targeting BCMA developed by Bristol-Myers Squibb (BMS), was approved by the FDA for the treatment of multiple myeloma. It became the fifth CAR-T cell therapy approved by the FDA and the first approved CAR-T cell therapy targeting a target other than CD19.
Cell therapy represented by CAR-T has become one of the most significant breakthroughs in the field of cancer treatment in recent years. As of April 16, 2021, there are a total of 2073 active cell therapy drug R&D pipelines worldwide, an increase of 572 over the same period in 2020, a growth rate of 38%.
Recently, Nature Reviews Drug Discovery published an article titled: The clinical pipeline for cancer cell therapies, summarizing the current clinical research and development pipelines for cancer cell therapies worldwide, the status of clinical trials, and the real-world information in clinical practice. data.

Steady growth of R&D pipeline
As of April 16, 2021, there are a total of 2073 active cell therapy drug R&D pipelines worldwide, an increase of 572 over the same period in 2020, a growth rate of 38%.
In different types of cell therapy, CAR-T continues to dominate, adding 299 new CAR-T drugs, an increase of 35% over the same period in 2020. Most CAR-T therapies (80%) are in the preclinical and clinical phase I stages.
TCR-T cell therapy added 80 new drugs, followed by NK/NK-T cell therapy with 67 new drugs.
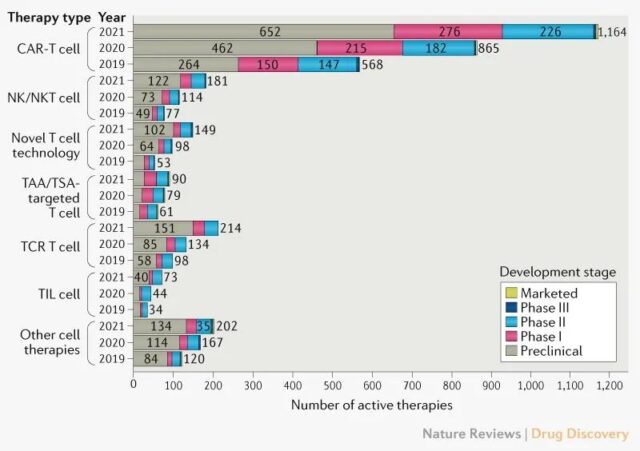
Changes in the cancer cell therapy pipeline
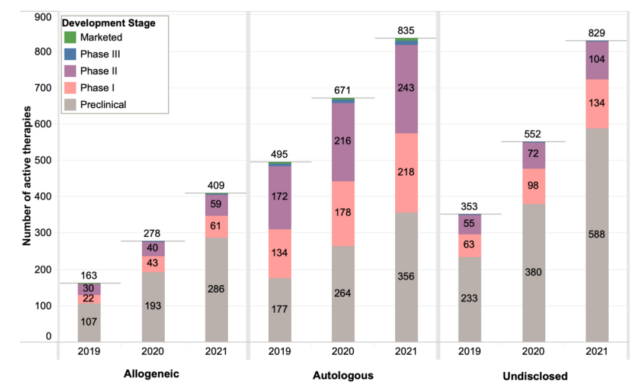
Most cell therapies (835 types) are of autologous origin, and the autologous cell therapies being developed are twice as high as allogeneic cell therapies. The number of allogeneic cell therapies in preclinical and clinical phase I has increased significantly in the past year (48% and 42%, respectively), but the growth rate has slowed compared with the same period last year (80% and 95%, respectively).
For cancer cell therapy targets, CD19, BCMA, and CD22 are still the most studied targets for hematological cancers, but the growth rate (15%, 23%, and 56%, respectively) compared with the previous year (51%, respectively) 83% and 80%) have slowed significantly.
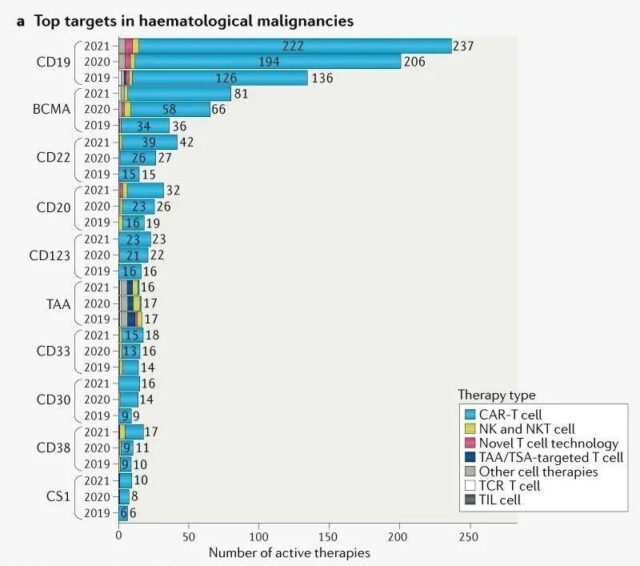
The main target of cell therapy for blood cancer
Solid tumors have always been a difficult point in cancer cell therapy. The target of solid tumor cell therapy has not changed much. The undisclosed tumor-associated antigens (TAA) are the most, followed by HER2, MSLN, GD2, EGFR, and GPC2/3. It is worth mentioning that the GPC2/3 target has grown rapidly since 2019, almost doubling every year, mainly for the treatment of liver cancer.
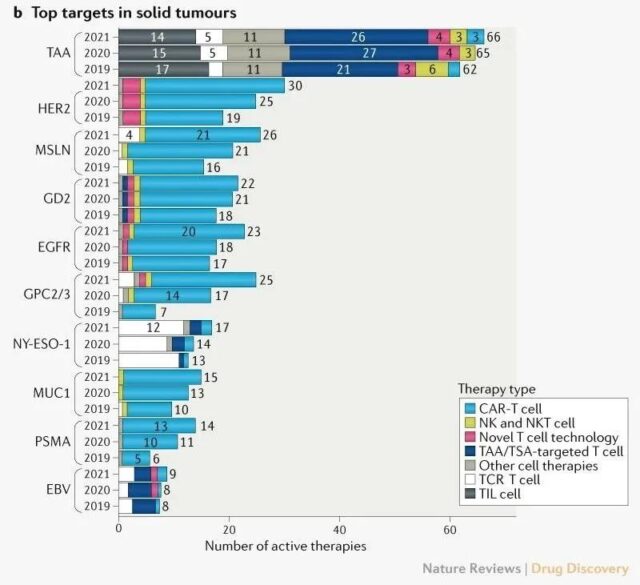
The main target of cell therapy for solid tumors
Clinical trial panorama
According to data obtained from ClinicalTrials.gov, as of April 2021, there are a total of 1,358 active cell therapy trials, which means an increase of 43% from 2020 to 2021, and an increase of 24% from 2019 to 2020.
Most of the growth comes from CAR-T cell clinical trials (increased by 83% since 2019), as well as other types of cell therapies, TCR-T, TIL, etc. After experiencing a sharp decline in 2020, NK/NKT has not yet fully recovered in 2021.
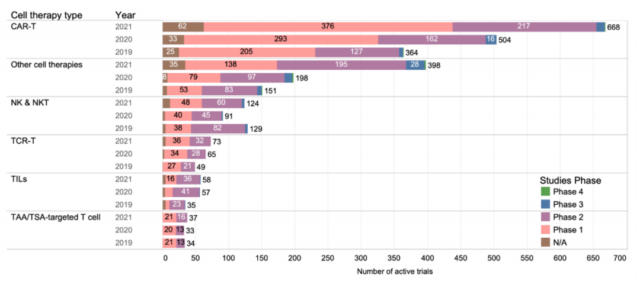
Similar to previous years, most clinical trials of cell therapy focused on hematological malignancies, solid tumors accounted for only 40%, and most of them are in the early clinical stage. This also reflects the inherent challenges of using cell therapy to treat solid tumors.
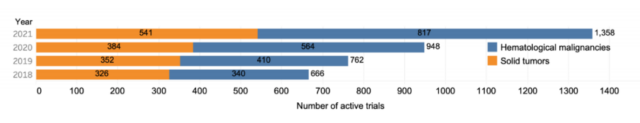
We found that all the top hospitals and universities conducting cell therapy trials are located in the United States (Figure 3), which shows that despite global interest in cell therapy, the United States is still the leader in trial management.
The top ten hospitals and research institutions for the number of clinical trials of cell therapy are: MD Anderson Cancer Center, Memorial Sloan Kettering Cancer Center, Mayo Medical Center, National Institutes of Health, City of Hope, University of Minnesota, Dana Farber Cancer Center, University of Pennsylvania, Sarah Cannon Institute, Texas Children’s Hospital.
These ten top hospitals and research institutions are all from the United States, which also shows that although the world is very interested in cell therapy, the United States is still in a leading position in the field of cell therapy clinical research.
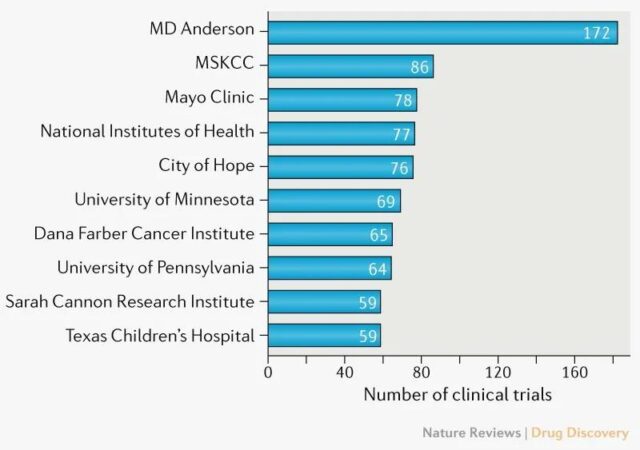
Global R&D pipeline
The United States and China continue to dominate the cell therapy R&D pipeline. The United States has 791 cell therapy R&D pipelines and China has 695 drugs. One order of magnitude more than other countries and regions.
In 2019 and 2010, the U.S. cell therapy R&D pipeline grew by 31% and 40%, respectively, while China’s growth rate was 40% and 69%, and China’s growth rate was even faster.
In the United States, most cell therapies are researched and developed by companies, with academia accounting for only 17%. China is more balanced, with 60% from companies and 40% from academia.
Real-world data on cell therapy
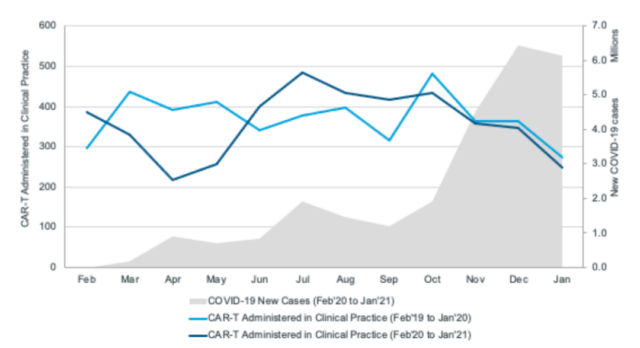
This article counts the number of cancer patients receiving CAR-T cell therapy in the United States and its changing trends. Between March 2020 and April 2020, the number of patients receiving CAR-T cell therapy dropped significantly, which coincided with the first wave of COVID-19 pandemic in the United States.
This may reflect the instability of the healthcare system during the initial COVID-19 pandemic, the reduction in the use of more selective therapies, the reduction of relevant medical staff and resources to support CAR-T cell therapy, and the reluctance of patients and medical staff to treat vulnerable cancer patients Exposure to a potentially high-risk hospital environment.
Sum up
Since the FDA approved the first CAR-T therapy in 2017, 5 cell therapies have been approved in less than 4 years, more than 2,000 cell therapies are being studied, and clinical development is continuing to develop steadily.
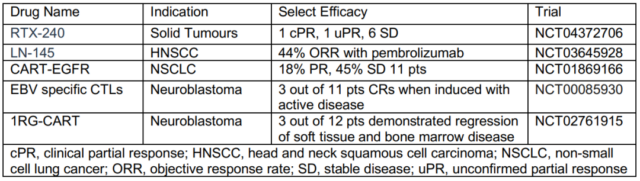
2017 is the first year of cell therapy. Now we have seen the vigorous development of cell therapy, a hundred flowers blooming, and more and more new targets and more and more indications are entering clinical trials. It is worth mentioning that solid tumors, which are difficult cell therapy problems, have begun to show some surprising clinical trial results.
In addition, as the world’s COVID-19 epidemic tends to ease, we can foresee a new growth in cell therapy clinical trials.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



