The fourth CAR-T cell therapy was approved!
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
The fourth CAR-T cell therapy was approved!
The fourth CAR-T cell therapy was approved! On February 5, 2021, US time, the US FDA announced that the fourth CAR-T cell therapy was approved for marketing!
The approved CAR-T cell therapy Breyanzi (lisocabtagene maraleucel) developed by Juno Therapeutics, a subsidiary of Bristol-Myers Squibb (BMS), is used to treat at least 2 other systemic therapies that do not respond or relapse after treatment. Certain adult patients with large B-cell lymphoma.

Image source: FDA official website
As the fourth FDA CAR-T cell therapy, Breyanzi approved this time is also indicated for hematological tumors. As the most common malignant lymphoma, diffuse large B-cell lymphoma (DLBCL)
Nearly 30-40% of patients cannot be relieved or relapsed after first-line treatment. Therefore, for this type of patients, new treatment methods are particularly urgent.
The newly approved Breyanzi is based on the final results of the TRANSCEND NHL 001 clinical trial. Among the more than 250 patients with DLBCL who have been treated with Breyanzi and who can be evaluated that are ineffective or relapsed in first-line treatment, 54% have achieved complete remission. The treatment data is pretty good.
In terms of side effects, Breyanzi treatment is also the same as other CAR-T cell therapies, which may produce cytokine storm and neurotoxicity risks. Therefore, when receiving Breyanzi treatment, it is necessary to pay close attention to the patient’s vital signs, but from now on Look, the side effects of CAR-T cell therapy can be reduced through the intervention of active professional medical personnel.
In October 2017, the FDA approved the listing of the world’s first CAR-T cell therapy. In the following December, the FDA approved the listing of the second CAR-T cell therapy. However, after nearly three years, we only In July 2020 ushered in the launch of the third CAR-T cell therapy. Fortunately, the fourth CAR-T cell therapy did not make us wait too long. In just a few months Today, we ushered in the launch of the fourth CAR-T cell therapy. Let’s take a look at the information on the CAR-T cell therapy that has been launched:
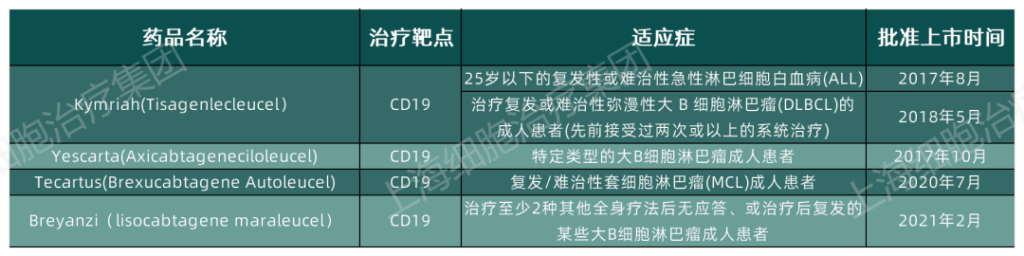
As of today, the FDA has approved a total of 4 CAR-T cell therapies for the market. These cell therapies are all aimed at hematological tumors, and they all use CD19 as the therapeutic target, and have achieved quite good therapeutic effects. It is precisely because of this that people have high hopes for CAR-T cell therapy, hoping that it will exert a stronger therapeutic effect in the field of tumor treatment.
We search for clinical trials using “CAR-T” and “tumor” as keywords in the global clinical trial centers. A total of 798 related clinical studies can be searched. From the perspective of the number of studies, China’s CAR-T therapy has already Surpassing the United States and becoming the country with the largest number of CAR T cell therapy clinical studies in the world today.
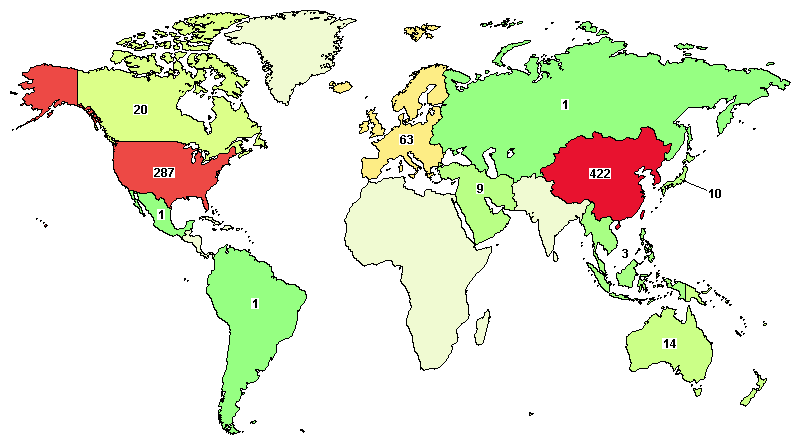
Image source: clinicaltrial official website
At the same time, in the screening of these 798 trials for Phase 2 and Phase 3 clinical trials, there are currently nearly 300 CAR-T therapy clinical studies. Many CAR-T cell therapies have a long history. Research time, and these CAR-T cell therapies are not only for hematological tumors, but also for solid tumors.
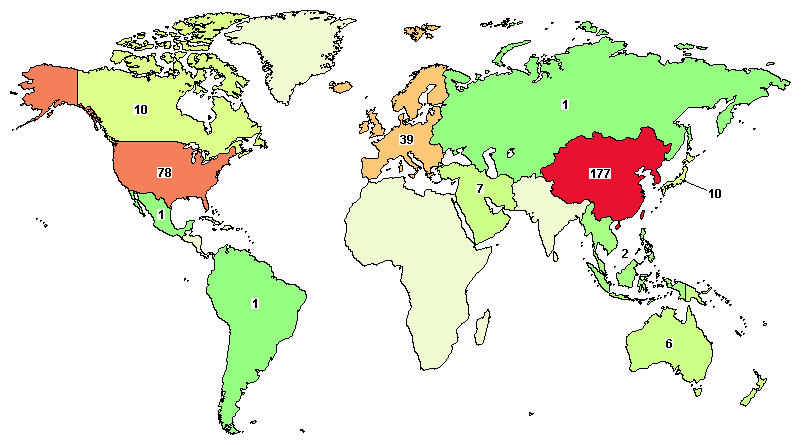
Image source: clinicaltrial official website
When analyzing these phase 2 and phase 3 clinical trials, we found that a variety of solid tumors are also undergoing CAR-T therapy. We can see CAR-T cell therapy from the experiment on the first page It is actively entering the field of solid tumor treatment, including: cervical cancer, esophageal cancer, ovarian cancer, endometrial cancer, breast cancer, pancreatic cancer liver metastasis, colorectal cancer, etc. These clinical studies are also continuing to recruit.
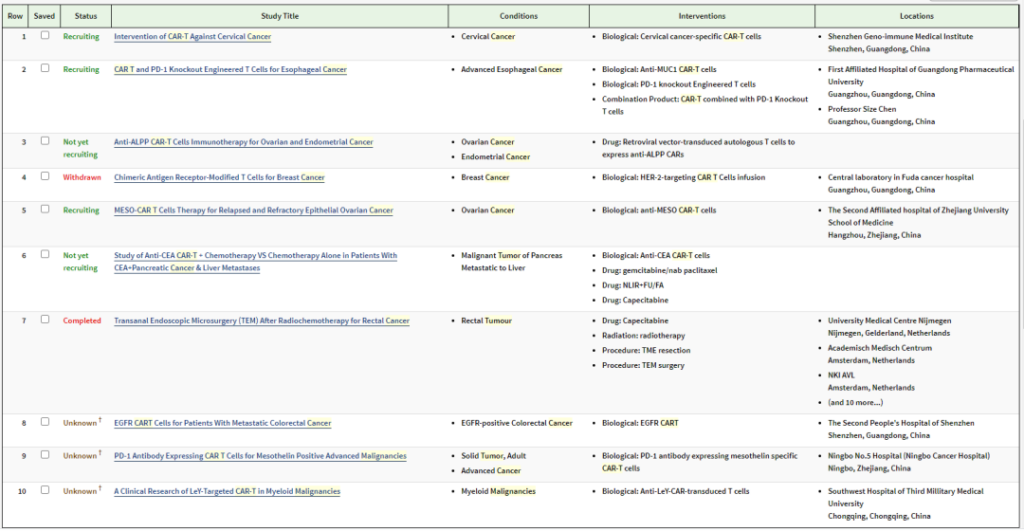
Image source: clinicaltrial official website
With the launch of CAR-T cell therapy in the United States, the approval of CAR-T cell therapy worldwide, especially in China, will continue to follow. China’s CAR-T cell therapy companies are also constantly developing new treatment technologies. I believe that so many clinical studies have entered Phase 2 and Phase 3. In 2021, we may be expected to usher in a major outbreak of CAR-T cell therapy. In the next 2 to 3 years, we will see more and more CAR-T cell therapies being approved for marketing, helping more patients fight cancer.
(source:internet, reference only)
Disclaimer of medicaltrend.org



