EU approved Libtayo for lung cancer and basal cell carcinoma
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
EU approved Libtayo immunotherapy for lung cancer and basal cell carcinoma!
EU approved Libtayo immunotherapy for lung cancer and basal cell carcinoma! Immunotherapy for lung cancer and basal cell carcinoma! The European Union approves Sanofi/Regeneron anti-PD-1 therapy Libtayo: treatment of 2 kinds of advanced cancer!
Sanofi and its partner Regeneron recently announced that the European Commission (EC) has approved the anti-PD-1 therapy Libtayo (cemiplimab) as a monotherapy for the treatment of two types of advanced cancers. Specifically:
(1) First-line treatment of advanced non-small cell lung cancer with high PD-L1 expression (TPS≥50%), metastatic or locally advanced disease, unsuitable for surgical resection or radical radiotherapy and chemotherapy, and tumor without EGFR/ALK/ROS1 aberrations (NSCLC) Adult patients; (
2) For adult patients with locally advanced or metastatic basal cell carcinoma (BCC) who are treated with a hedgehog pathway inhibitor (HHI) but have progressed or are intolerant to the drug.
In 2019, Libtayo was approved for the first time in the European Union for the treatment of patients with advanced skin squamous cell (CSCC), specifically for: patients with metastatic CSCC and locally advanced CSCC patients who are not suitable for radical surgery or radical radiotherapy.
Up to now, Libtayo has been approved to treat 3 kinds of advanced cancers (CSCC, NSCLC, BCC) in the European Union. CSCC and BCC are the two most common types of skin cancer. Libtayo is the first drug approved for the treatment of advanced CSCC and the first immunotherapy approved for the treatment of advanced BCC.
Lung cancer is the leading cause of cancer deaths worldwide, with more than 2 million new cases diagnosed each year. Approximately 85% of lung cancers are non-small cell lung cancer (NSCLC), and it is estimated that 25%-30% of cases have high PD-L1 expression (PD-L1 positive is detected in ≥50% of tumor cells). In recent years, although immunotherapy has changed the treatment of patients with advanced NSCLC, there is still a need to optimize the identification and treatment of patients with high PD-L1 expression.
EC’s approval of Libtayo’s first-line treatment for advanced NSCLC with high PD-L1 expression is based on data from the key Phase 3 EMPOWER-Lung 1 study. The study was carried out in locally advanced or metastatic NSCLC patients with ≥50% tumor cells expressing PD-L1. Data show that compared with platinum-containing dual-effect chemotherapy, Libtayo first-line treatment significantly prolonged overall survival (OS).
Although a very high proportion of patients in the chemotherapy group (crossover rate 74%) switched to the Libtayo group after the disease progressed, Libtayo is still superior to chemotherapy in prolonging OS: (1) In the entire study population, Libtayo will die compared with chemotherapy The risk was reduced by 32%, with a median OS of 22 months in the Libtayo treatment group and 14 months in the chemotherapy group. (2) In patients with PD-L1 expression ≥50%, Libtayo reduced the risk of death by 43% compared with chemotherapy. The median OS in the Libtayo treatment group has not yet reached, and the chemotherapy group is 14 months.
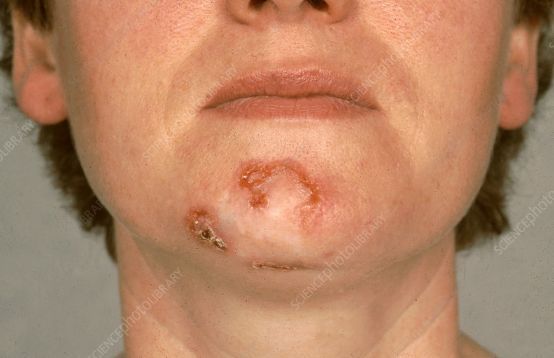
Basal cell carcinoma (BCC, image source: sciencephoto.com)
Basal cell carcinoma (BCC) is the most common type of skin cancer in the world. Although the vast majority of BCC is found at an early stage and can be easily cured by surgery and radiation therapy, there are also a small number of tumors that progress to advanced stages and penetrate deep into the surrounding tissues (local progression), which is more difficult to treat.
The EC’s approval of Libtayo for the treatment of locally advanced or metastatic BCC is based on data from a phase II clinical trial (NCT03132636), the results of the largest prospective clinical trial conducted on these patients to date.
The results showed: (1) In patients with metastatic BCC (mBCC), the median follow-up was 9.5 months, the overall response rate (ORR) was 21%, and the median duration of response (DOR) had not yet reached (9-23+). All patients had DOR ≥ 6 months; (2) In the locally advanced BCC (laBCC) cohort, the median follow-up was 15.1 months, the ORR was 31%, the median DOR has not yet been reached, and it is estimated that 85% of patients in remission have DOR ≥ At 1 year, the one-year disease progression-free survival rate was 57%, and the one-year survival rate was 92%.
Libtayo belongs to the anti-PD-(L)1 inhibitor, which is currently a high-profile tumor immunotherapy. It aims to use the body’s own immune system to fight cancer and block the PD-1/PD-L1 signaling pathway to make cancer. Cell death has the potential to treat many types of tumors. Libtayo is a fully human monoclonal antibody that targets the immune checkpoint receptor PD-1 on T cells. By binding to PD-1, Libtayo has been shown to prevent cancer cells from inhibiting T cell activation through the PD-1 pathway.
Libtayo uses Regeneron’s patented Velocimmune technology platform to create and optimize it. It is currently under joint development under the framework of a global cooperation agreement between Regeneron and Sanofi for the treatment of various types of cancer. Libtayo’s extensive clinical projects focus on refractory cancers, including skin cancer, cervical cancer, solid tumors and blood cancers.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



