The fourth CAR-T therapy was approved to treat large B-cell lymphoma
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
The fourth CAR-T therapy was approved to treat large B-cell lymphoma
The fourth CAR-T therapy was approved to treat large B-cell lymphoma, with better efficacy and safety.
Large B-cell lymphoma is the most common type of non-Hodgkin’s lymphoma, and Breyanzi is a targeted CAR-T therapy.
Recently, the US FDA announced the approval of the new CAR-T therapy Breyanzi (lisocabtagene maraleucel) for the treatment of certain types of adult patients with large B-cell lymphoma (DLBCL) who have not responded to at least 2 other systemic treatments, or Relapse occurred after treatment.
Breyanzi is a CAR-T cell therapy targeting CD19 antigen, and it is also the fourth CAR-T therapy for specific types of non-Hodgkin’s lymphoma. However, the drug has not been approved to treat patients with primary central nervous system lymphoma.
▌DLBCL treatment problem: easy to drug resistance relapse

Large B-cell lymphoma (DLBCL) is the most common type of non-Hodgkin’s lymphoma (NHL), accounting for 40% of B-cell NHL, and China has an estimated 50,000 new cases each year. DLBCL disease progresses rapidly and is highly aggressive. It is characterized by a rapid increase in the number of malignant B cells in lymph nodes, spleen, liver, bone marrow or other organs.
Although there are various types of chemotherapy and targeted drug combination treatment options, about one-third of patients do not respond to the initial therapy or relapse. The survival period of chemotherapy rescue treatment is usually less than 6 months, and the survival period is shorter.
In June and July last year, the FDA approved the selective nuclear export protein inhibitor Xpovio (selinexor) and Monjuvi combination therapy for the treatment of patients with large B-cell lymphoma.
Up to now, the US FDA has approved two CAR-T therapies Yescarta and Kymriah for the treatment of patients with relapsed/refractory large B-cell lymphoma (including the most common diffuse large B-cell lymphoma).
The approval of the new therapy has largely alleviated the dilemma of resistance or ineffectiveness of DLBCL treatment. However, for patients who cannot tolerate strong chemotherapy or are not suitable for autologous stem cell transplantation and CAR-T cell therapy, the prognosis is particularly poor, and more treatment options are still needed.
▌CAR-T therapy: Breyanzi
Breyanzi is an autologous CAR-T therapy targeting CD19 antigen. It consists of prepared and purified CD8+ and CD4+ T cells in a specific ratio (1:1), which facilitates better control of the side effects of cell therapy and improves product safety Sex.
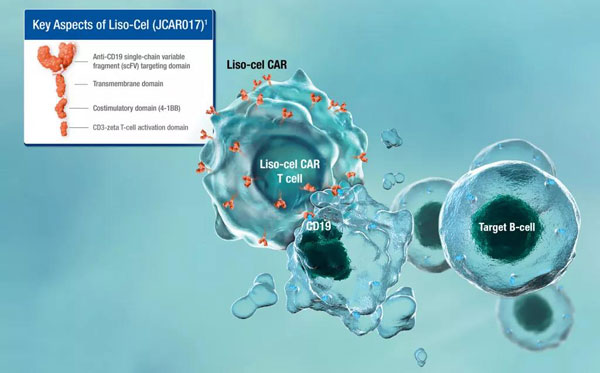
Previously, the FDA had granted Breyanzi the titles of Regenerative Medicine Advanced Therapy (RMAT), Orphan Drug, and Breakthrough Therapy.
▌The highest mitigation rate and the best safety! The test data is amazing!
This approval is supported by positive data from a multicenter clinical trial called TRANSCEND NHL 001, which is the largest CD19-targeted CAR-T therapy to date.
The trial enrolled 344 patients with large B-cell lymphoma over 18 years of age who had previously received 2 or more therapies and had undergone leukocyte separation.
The study reached the primary and secondary endpoints. The primary endpoint is the objective response rate (ORR). Secondary endpoints include complete response rate (CR), duration of response (DOR), progression-free survival (PFS), and overall survival (OS).
The results show that:
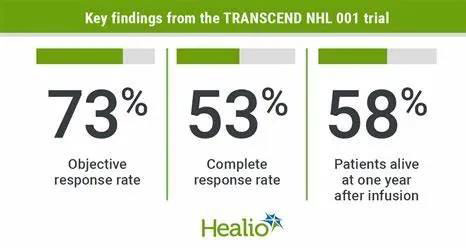
- 1. Among the 256 patients whose efficacy can be evaluated, the objective remission rate of Breyanzi treatment group was 73%. Among them, more than half of the people achieved complete remission, with a CR of 53%, and the time to first remission or partial remission was a median of 1 month.
- 2. The median follow-up for 12 months has not reached the median time of remission, but at 6 months, 60.4% of patients still responded, and 54.7% of patients continued to respond at 12 months.
- 3. The median PFS was 6.8 months, and the PFS rate at 1 year was 44.1%. The median OS was 21.1 months, and the OS was 57.9% at one year.
The key is that compared with the approved CAR-T therapy Yescarta, Kymriah trial data, Breyanzi treatment group patients have a higher remission rate and the best safety. During the course of treatment, only 2% (6 cases) of patients developed grade 3/4 cytokine release syndrome (CRS).
This approval provides a new treatment option for patients with certain cancers that affect the blood, bone marrow and lymph nodes, and is also another milestone in the field of cellular immunotherapy!
▌A list of approved CAR-T cell therapies:
In August 2017, the US FDA announced that the CAR-T therapy Kymriah was approved for marketing, for the treatment of relapsed or refractory acute lymphoblastic leukemia (ALL) patients under the age of 25. This is the world’s first approved CAR-T therapy.
In May 2018, Kymriah was approved for the second indication for the treatment of adult patients with relapsed or refractory diffuse DLBCL (previously received two or more systemic treatments).
In October 2017, the CAR-T therapy Yescarta was approved by the US FDA for the treatment of adult patients with specific types of large B-cell lymphoma.
Yescarta is the first CAR-T therapy approved by the U.S. FDA for specific non-Hodgkin’s lymphoma and the second approved CAR-T therapy.
In July 2020, the FDA accelerated the approval of the CAR-T cell therapy Tecartus for the treatment of adult patients with relapsed/refractory mantle cell lymphoma (MCL). This is the third CAR-T cell therapy approved globally.
(source:internet, reference only)
Disclaimer of medicaltrend.org



