Lymphoma: CAR-T therapy better and more effective than chemotherapy
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Lymphoma: CAR-T therapy better and more effective than chemotherapy
Lymphoma: CAR-T therapy better and more effective than chemotherapy. It was confirmed for the first time that CAR-T therapy is better and more effective than chemotherapy in treating this type of cancer!
Lymphoma is one of the top ten malignant tumors with the highest mortality rate. There are about 93,000 new patients with lymphoma every year in China, and more than 50,000 people die from this disease every year.
Lymphoma is one of the top ten malignant tumors with the highest mortality rate. In fact, lymphoma is not an incurable disease. From the data point of view, the survival rate of lymphoma in Europe and the United States can reach 70% to 80%, while China is relatively low, reaching about 30% to 40%.
Today we will focus on relapsed/refractory peripheral T-cell lymphoma (PTCL).
“Hard point” in non-Hodgkin’s lymphoma: peripheral T-cell lymphoma
Non-Hodgkin’s lymphoma is divided into two categories: B cell type and T/NK cell type. Among them, peripheral T/NK cell lymphoma is a “suffering household” among non-Hodgkin’s lymphomas, and the five-year survival rate is hovering around 30%.
Peripheral T-cell lymphoma (PTCL) is a group of heterogeneous and highly aggressive non-Hodgkin’s lymphomas (NHL), accounting for about 10% to 15% of non-Hodgkin’s lymphomas in European and American countries, while some countries’s Around 21.4%.
Since 2009, the U.S. Food and Drug Administration (FDA) has approved three medications for PTCL patients:
- Pratrixa (Pralatrexate)
- Romidepsin (Istodax)
- Belinostat (Beleodaq)
However, the first-line treatment effect of peripheral T-cell lymphoma is not satisfactory. 30% of patients will develop resistance, 18% of patients will relapse within 2 years, 12% of patients will relapse within 7 years, and only 40% of patients will Get long-term survival. Therefore, treatment after recurrence is particularly important! Many medical researchers have turned their attention to the hottest chimeric antigen receptor T (CAR-T) cell therapy in immunotherapy.
CD4-CAR treatment was approved by FDA, Orphan Drug for Peripheral T-cell Lymphoma
On August 12, 2016, the gene therapy company iCell announced that its CD4CAR for the treatment of peripheral T-cell lymphoma (PTCL) has obtained the orphan drug designation granted by the FDA. It is understood that CD4CAR is a target protein CD4 chimeric antigen receptor It is specially developed to transform T cells (CD4CAR).

According to clinical experiments, most peripheral T-cell lymphomas are CD4 positive, but few are CD8 positive, CD4 and CD8 are double positive or have NK cell immunophenotype. To diagnose peripheral T-cell lymphoma or its special subtypes, it is also necessary to be obtained by a professional hematology pathologist based on the appropriate biopsy pathology and immunophenotype.
It is reported that CD4CAR is derived from the development of CD4+. CAR-T has shown targeting, killing activity and durability in the clinical application of cells, which injects new solutions for tumor diseases such as lymphoma.
The qualification of this drug design establishes its status as an orphan drug, and encourages more research and development of related drugs and biological agents for the safety and effectiveness of prevention, diagnosis and treatment methods.
First time! Peripheral blood T-cell lymphoma: a clinical trial of human CD4CAR
CD19 CAR-T cell therapy has successfully treated acute lymphoblastic leukemia. However, the treatment of Sezary syndrome [invasive form of skin peripheral T-cell lymphoma (PTCL)] remains a challenge. Although patients with Sezary syndrome usually receive multiple treatments during their disease progression, the prognosis is poor, with a 5-year survival rate of only 24%. Therefore, it is very important to establish a new PTCL treatment method.
In November 2019, a prospective study published in the internationally renowned “Blood” magazine verified the safety and effectiveness of CD4 CAR-T cells in patients with Sezary syndrome.
According to the results of preclinical studies, CD4 CAR-T cells are an effective method for the treatment of peripheral T-cell lymphoma. Patients participating in the Phase I dose escalation trial showed a significant response to CD4 CAR-T cell therapy.
Analysis of a typical case
It is worth noting that the 54-year-old patient diagnosed with Sezary syndrome has been completely relieved by CD4 CAR-T cell therapy.
Before admission, he had suffered from erythroderma, itching and skin scaly symptoms for more than 10 years, and he was resistant to various chemotherapy regimens. Before starting the CAR treatment, the patient’s skin had extensive leukemia infiltration (Figure 1A), confirmed by skin biopsy (Figure 1A, 1B) bone marrow and blood contained 50% leukemia cells (Figure 1C).
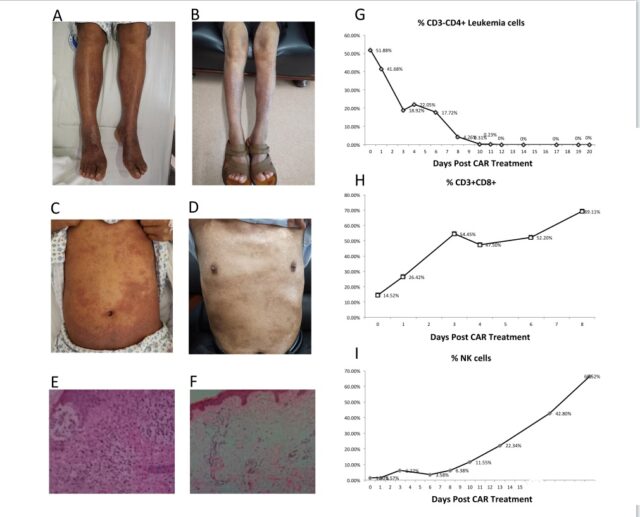
The patient received a single dose of CAR-T cells with a total dose of 3×10^6/kg, and then was given fluconazole and valacyclovir to prevent infection. Since the patient received CD4 CAR-T cell transfusion, the percentage of CAR-T cells and NK cells in the peripheral blood (Figure 1D) has been increasing (Figure 1E). On the 13th day, the patient was in complete remission and the percentage of leukemia cells in the blood dropped to zero. On the 28th day, the appearance of the skin changed drastically from before the treatment. Significant skin regeneration was observed on the patient’s legs (Figure 1F). Flow cytometry of bone marrow and peripheral blood confirmed the absence of tumor cells.
In addition, skin biopsies at multiple sites after CAR treatment showed the absence of leukemia infiltration (Figure 1G). The patient was discharged from the hospital without other medications. Throughout the course of treatment, the patient had no toxic reactions related to CAR-T cell therapy.
This first human clinical trial proved that CD4 CAR-T cell therapy can effectively treat patients with refractory Sezary syndrome. CD4CAR can eradicate leukemia blasts and exert a better and more significant tumor killing effect than traditional chemotherapy.
At present, relapsed and refractory peripheral T-cell lymphoma is still a clinical focus and difficulty, and traditional salvage chemotherapy has limited efficacy. However, the therapeutic potential of CAR-T therapy in peripheral T-cell lymphoma is endless.
It is believed that with the development of related research, CAR-T therapy will continue to expand its indications, bringing new treatment options and hope for cure to more cancer patients!
(source:internet, reference only)
Disclaimer of medicaltrend.org



