Immunomodulatory effects of targeted anticancer drugs
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Immunomodulatory effects of targeted anticancer drugs
Immunomodulatory effects of targeted anticancer drugs. In the past thirty years, a large number of preclinical and clinical data have shown that almost all targeted anti-cancer drugs have more or less immunostimulatory or immunosuppressive effects, thereby affecting the therapeutic effect.
The immunomodulatory activity of targeted anti-cancer drugs can be derived from the interaction of drugs with cancer cells and the ability of drugs to interact with immune cells and change their functions.
The general mechanisms of these immunomodulatory targeted anticancer treatments can involve direct (ie, increase in specific effects) or indirect (ie, decrease in antagonism) pathways. For example, targeted anti-cancer drugs can mediate immunostimulatory effects by promoting the secretion of pro-inflammatory cytokines or by limiting the release or activity of immunosuppressive factors. In addition, targeted anti-cancer drugs can trigger the high immunogenicity of cancer cell death to initiate the so-called cancer immune cycle or selectively promote the depletion of immunosuppressive cells, such as regulatory T (Treg) cells.
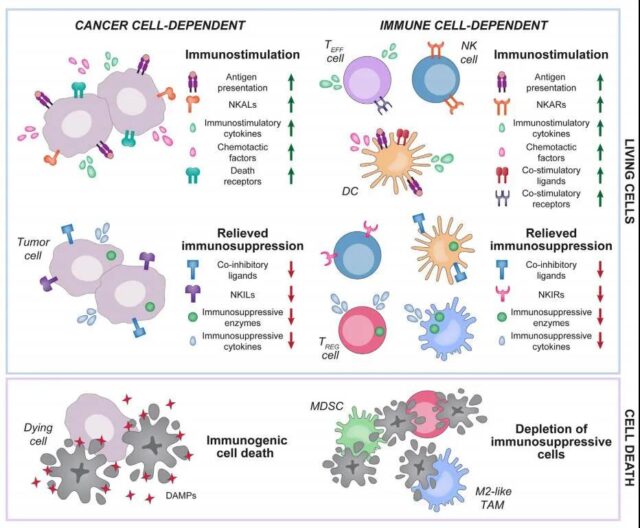
The ability of targeted anti-cancer drugs to mediate immunomodulatory effects provides a strong theoretical basis for the development of combination programs involving immunotherapy. Below, we jointly review the latest mechanism progress of the main immunomodulatory effects of the US Food and Drug Administration (FDA) approved and experimentally targeted anticancer drugs, so as to obtain the combination of these drugs and immunotherapy for higher clinical efficacy Potential way.
CDK inhibitor
Cyclin-dependent kinases (CDKs) are a family of serine/threonine kinases that regulate cell cycle progression and other cellular processes, including DNA repair, transcription, and metabolism. CDK has attracted widespread attention as a potential target for the development of new anti-cancer therapies. Recently, three different CDK4/CDK6 inhibitors have been approved for HR+ breast cancer patients. More and more evidences show that CDK4/CDK6 inhibitors and other CDKs inhibitors can not only prevent the proliferation of malignant cells, but also mediate a variety of immunomodulatory effects.

First, the inhibition of CDK4/CDK6 can mediate immunostimulatory effects by promoting the exposure of MHC class I molecules on the surface of tumor cells; secondly, CDK4/CDK6 inhibitors can also mediate immunostimulatory effects by promoting the secretion of pro-inflammatory cytokines , Such as type III interferon and CC chemokine ligand 5 (CCL5); finally, various CDK4/CDK6 inhibitors mediate a variety of immunostimulatory effects through direct interaction with immune cells, these effects include (1) effects T (TEFF) cells activate the nuclear factor 1 (NFATC1) signal and interleukin-2 (IL-2) secretion of activated T cells, (2) inhibit immunosuppressiveness by inhibiting DNA methyltransferase 1 (DNMT1) Treg cells are subsequently blocked by CDK inhibitor 1A (CDKN1A).
The immunostimulatory activity of CDK4/CDK6 inhibitors can be offset by their ability to up-regulate the immunosuppressive molecule CD274 (PD-L1) through transcription and post-translational mechanisms. Therefore, immune checkpoint inhibitors (ICI) targeting PD-L1 or its receptor (PD-1) are ideal combination partners for CDK4/CDK6 inhibitors.
In addition, CDK4/CDK6 inhibitors can also be combined with other targeted anti-cancer drugs to obtain better immune stimulation and superior efficacy. For example, the MEK inhibitor trametinib and palbociclib synergistically induce SASP-dependent vascular responses, and achieve tumor infiltration through immune effector cells.
KRAS and PI3K inhibitors
Some tumors are driven by gain-of-function mutations or deletions in KRAS, PI3KCA, or B-Raf proto-oncogene (BRAF) and phosphatase and tensin homolog (PTEN), which are generated through the signal pathway of AKT1, MTOR, or MEK Sexual mitogenic signal. At present, although BRAF, PI3K, MTOR and MEK inhibitors have been successfully developed into targeted anticancer drugs approved by the FDA, KRAS inhibitors have not yet been licensed for use in humans, and the development of AKT1 inhibitors is also in its infancy. Interestingly, many of these drugs not only limit the mitotic signal in cancer cells, but also mediate a series of treatment-related immunomodulatory effects.

Activation of KRAS and BRAF mutations in malignant cells supports the establishment of an immunosuppressive microenvironment through a variety of mechanisms. Therefore, BRAF and MEK inhibitors (including the FDA-approved drugs vemurafenib, dabrafenib and trametinib) mediate various cancer cell-dependent immunostimulatory effects, including (1) up-regulating TAAs; (2) improving the antigen presentation of MHC class I molecules; (3) Induce ICD; (4) Secret TH1 cytokines, such as CXCL9 and CXCL10; (5) Down-regulate immune regulatory factors, including IL8, VEGFA, and SPP1.
On the other hand, when the tumor still progresses after treatment with KRAS, BRAF or MEK inhibitors, loss of antigen presentation, TEFF cell failure, and immunosuppressive cell infiltration are commonly observed in preclinical studies. To a certain extent, this reflects the key role of MEK signal in initiating the expansion of primitive T cells and the role of protecting tumor-infiltrating CD8+CTL from depletion. This potentially harmful effect can be avoided or reversed (at least in part) with a variety of drugs other than PD-1/PD-L1 blockers. These drugs include (but are not limited to) (1) CTLA-4 or TIM-3 Blockers; (2) immunostimulatory molecules containing CD40, OX40, 4-1BB and Toll-like receptor 7 (TLR7) agonists, as well as recombinant IL15 and FLT3LG; (3) CDK4/CDK6 inhibitors; (4) enhancement Prebiotics for general immunity, such as inulin.
In short, the deregulated KRAS and PI3K signals mediate a powerful carcinogenic effect and at the same time facilitate immune evasion. Consistent with this concept, inhibitors of KRAS and PI3K signaling usually exert powerful and treatment-related immunostimulatory effects, although under certain circumstances, T cell failure appears as a resistance mechanism, which can be achieved through a variety of immunotherapies other than ICIs get over.
DDR and apoptosis targeted drugs
Certain malignant tumor cells show a high degree of dependence on cDNA damage repair (DDR) mechanisms or powerful anti-apoptotic signals. This dependence has been used to develop targeted anticancer drugs based on the principle of synthetic lethality. Nowadays, poly(ADP)-ribose polymerase 1 (PARP1) inhibitors are licensed to treat DDR-deficient cancers, such as breast and ovarian tumors with BRCA1 or BRCA2 mutations. Similarly, BCL2 inhibitors are approved for patients with CLL or small lymphocytic lymphoma (SLL). All these DDR-targeted drugs have shown an immunomodulatory effect that mediates treatment relevance.

Various FDA-approved and experimental PARP inhibitors have been shown to promote the secretion of type I interferon in a variety of tumor cells, in some cases, accompanied by the release of T cell chemoattractants (including CXCL10), and DC Paracrine activation ultimately leads to the up-regulation of MHC class II molecules and costimulatory ligands to support T cell activation.
In addition, regardless of BRCA1/2 status, PARP inhibitors are associated with the up-regulation of PD-L1 in various cancer cells, which may be the result of type I interferon or interferon signal transduction. In fact, various ICIs have been successfully used to enhance the therapeutic activity of PARP inhibitors in preclinical tumor models.
ATM has been shown to cooperate with PARP1 to activate a non-classical STING-dependent program that focuses on NF-kB-driven type I interferon and IL6 secretion. In addition, ATM, independent of its role in DDR, drives breast cancer cells to secrete IL8, and ultimately promotes disease progression through autocrine or paracrine mechanisms. Therefore, the ATM/NF-kB signal axis is not only a target to achieve local immune stimulation, but also a target to combat the internal disease progression pathway of cancer cells.
Another DDR-related kinase, ATR, has been shown to mediate powerful immunosuppressive effects. Therefore, ATR inhibitors not only enhance the CGAS signal and subsequent type I interferon response (involving CCL5 and CXCL10), but also enhance the antigen of MHC I molecules. The presentation is ultimately conducive to the tumor infiltration of DC, the repolarization of TAM in the direction of immune stimulation, and the T cell-dependent anti-cancer immunity.
DDR-driven BCL2 can inhibit apoptosis, one of the main players, p53 has gene or function loss in a variety of malignant tumors, which is related to local immunosuppression. Other immunostimulatory effects related to p53 drug reactivation include (but are not limited to) (1) down-regulation of immunomodulatory cytokines related to tumor progression, such as IL6; (2) up-regulation of NKALs; (3) establishment of immunostimulatory SASP Related to cell senescence, the SASP facilitates the recruitment and activation of NK cells, TEFF cells and macrophages.
HER2, EGFR, VEGFA and TGF-β inhibitors
In the past two decades, many monoclonal antibodies and TKIs have been developed to target these proteins or their binding partners. Some of these molecules, including HER2-, EGFR- and VEGFA-specific drugs, have been licensed for use in multiple drugs. Kind of tumor indications. The safety and effectiveness of TGF-β receptor or ligand inhibitors are still being examined, and all these drugs have been shown to mediate treatment-related immunomodulatory effects.

The HER2-targeted monoclonal antibody seems to be involved in a certain degree of CD8+ and CD4+ T cell-dependent HER2-specific immune response, which at least partially reflects the improvement of antigen presentation on MHC class I and II molecules, and the MYD88-dependent immune stimulation , Which ultimately leads to increased antigen processing and presentation by DC. A variety of immunotherapeutic agents, including DC-based vaccines, TLR2 agonists, recombinant IL21, and PD-L1 blockers can be used to amplify the efficacy of HER2-targeted mAbs while stimulating powerful anti-tumor immunity.
Various EGFR targeting agents can improve the MHC class I antigen presentation of cancer cells, and facilitate the uptake of tumor materials by DCs and the ability to stimulate T cells and promote NK cell activation without additional immune stimulation signals.
In addition, a variety of EGFR-targeted TKIs effectively reduced the expression of PD-L1 in NSCLC cells, reflecting their ability to block NF-kB and IL6 signaling. Consistent with this immunomodulatory feature, TME of NSCLC exposed to EGFR-targeted drugs showed increased abundance of DC and CD8+ Teff cells, as well as depletion of Treg cells and M2 TAM.
VEGFA monoclonal antibody and drugs targeting VEGFA receptor mediate immunostimulatory effects independent of ADCC and ADCP, which to a large extent reflects the key role of VEGFA in establishing cancer-related immunosuppression. For example, VEGFA signal is directly involved in Treg cell expansion and co-inhibitory receptor expression, including CD8+CTL PD-1. In addition, transcriptome analysis showed that VEGFA levels in breast cancer patient biopsies and preclinical models of breast cancer and colorectal cancer were negatively correlated with the characteristics of CD8+CTL invasion. Finally, in mouse models of ovarian cancer, the accumulation of immunosuppressive cells (including TAM) is related to the resistance of VEGFA targeting antibodies.
TGF-β is best known for its ability to up-regulate PD-L1 of malignant cells and myeloid cells in TME, promote PD-1 expression of T cells, and facilitate the expansion of Treg cells and MDSCs, and ultimately lead to local and systemic Immunosuppressive. In addition, TGF-β is closely related to the establishment of immune rejection. Through this process, cancer cells combine with cancer-related fibroblasts to prevent immune effector cells from infiltrating the tumor.
Inhibition of TGF-β or its receptor can solve immune rejection and improve the antigen presentation of malignant cells, and finally produce an effective anti-tumor immune response, which can work with ICIs to eliminate tumors and establish protective immune memory. In addition, although TGF-β plays a central role in the establishment of tumor-related immunosuppression, a combination of one or more ICIs is required to fully realize the clinical potential of TGF-β inhibitors. In this case, the order of treatment may be a key determinant of success. In fact, by supporting matrix metallopeptidase 9 (MMP9)-driven PD-L1 cleavage, pre-TGF-β inhibition seems to promote resistance to PD1 blockers at least to some extent. On the contrary, once PD-1 resistance is acquired, the administration of TGF-β inhibitors will produce a powerful therapeutic effect.
Others
The list of approved or under development targeted anti-cancer drugs is increasing. Such a list includes:
- The relatively selective ALK receptor tyrosine kinase (ALK);
- AXL receptor tyrosine kinase (AXL);
- Bruton tyrosine Acid kinase (BTK);
- zeste homolog 2 enhancer (EZH2);
- histone deacetylase (HDAC);
- isocitrate dehydrogenase 1 (IDH1);
- Janus kinase 1 (JAK1);
- MET proto-oncogene, Receptor tyrosine kinase (MET);
- ret proto-oncogene (RET);
- ROS proto-oncogene 1, receptor tyrosine kinase (ROS1);
- smooth frizzled receptor (SMO) and exportin 1 (XPO1) inhibitors ;
- Monoclonal antibodies specific for CD20, CD22, CD33, CD52, CD58;
- IL2 receptor subunit α (CD25); IL3 receptor subunit α (IL3RA);
- tumor-associated calcium signal transducer 2 (TROP2);
- Multi-target TKIs.
Interestingly, most of these clinically used drugs mediate at least some degree of immunomodulation, providing a theoretical basis for the design of combination treatment regimens involving ICIs.

Outlook and outstanding issues
Targeted anti-cancer drugs mediate powerful immunomodulatory effects. They either benefit the positive immune response or counteract negative immunosuppressive pathways. Therefore, the combination therapy of targeted anti-cancer drugs, ICIs and other immunotherapeutic strategies shows Very promising application. However, the biological basic research on immunomodulation of targeted anti-tumor drugs has just started, and there are still some problems to be solved in this respect.
First, what is the actual specificity of these drugs for their molecular targets? Imatinib was designed to target the kinase domain of ABL1 to inhibit the constitutive signaling of the BCR-ABL1 chimera, and it was found that it inhibited a variety of clinically relevant kinases. In a similar way, abemaciclib is believed to inhibit CDK4/CDK6, but it has been shown to target other CDKs and CDK-unrelated kinases. Therefore, although in some cases, the immunomodulation of targeted anti-tumor therapy is clearly derived from targeted effects, in many other cases it is derived from alternative (usually uncharacterized) other participating molecules. Especially when immune cells are directly involved, it is very urgent to identify these alternative targets.
Second, when immune regulation actually originates from the suppression of expected molecular targets in malignant cells, what is the exact molecular mechanism that works? There is no specifically designed targeted anti-cancer drugs to mediate immune regulation, which means that various proteins that support tumorigenesis also affect the ability of tumor cells to transmit immune stimulus or immune suppression signals. For example, active CDK4/CDK6 signals limit the ability of DNMT1 to drive the expression of endogenous retroviral elements, otherwise it will promote the secretion of type III IFN. This pathway can be used clinically by CDK4/CDK6 inhibitors. Accurately identifying similar pathways induced by targeted anticancer drugs will provide a variety of additional targets that can be used to enhance clinically significant tumor-specific immune responses. This is especially important when the immunomodulatory activity of a drug is multifunctional and involves beneficial and harmful effects, which can be used differently by targeting downstream signal transducers.
Third, in a specific environment, what are the key factors for the difference in immunomodulatory effects mediated by certain targeted anti-cancer drugs? Most drugs are related to immune stimulation and immunosuppressive effects, depending on the specific experimental scenario. However, while in some cases, the source of this difference is obvious, in many other cases, the underlying mechanism remains unclear. In the latter case, experimental variables such as drug concentration, precise medium composition and time may have unexpected effects on biological results. In addition, potentially overlooked and/or difficult-to-control heterogeneous genetic, epigenetic or metabolic sources may have a critical impact, especially in the body. It is necessary to further study the impact of tumor heterogeneity on the immunomodulatory effects of targeted anticancer drugs.
Fourth, how to use conventional chemotherapy and radiotherapy to maximize the ability of targeted anti-tumor drugs to drive clinically significant tumor-specific immune responses? It is clear (at least some) that targeted anticancer drugs are promising combination partners for various immunotherapies, including ICIs. However, whether the efficacy of these combination therapies can be further improved by conventional chemotherapy or RT has not been fully studied.
In short, only by understanding the molecular pathways of targeted anti-cancer drugs that mediate clinically relevant immunomodulation, and conceiving a multi-modal treatment plan with good immunostimulatory activity, can we further expand the use of targeted anti-cancer treatments to obtain clinical benefits for tumor indications. Quantity.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



