Nature: Why anticancer drugs always failed in human trials?
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Nature: Why anticancer drugs always failed in human trials?
Nature: Why anticancer drugs always failed in human trials? Cancer is a common disease that seriously threatens human health. In recent years, despite the fruitful results in the development of new anti-tumor drugs, more drugs have only stopped in clinical trials. The root cause is the lack of suitable in vivo and in vitro platforms for preclinical evaluation.
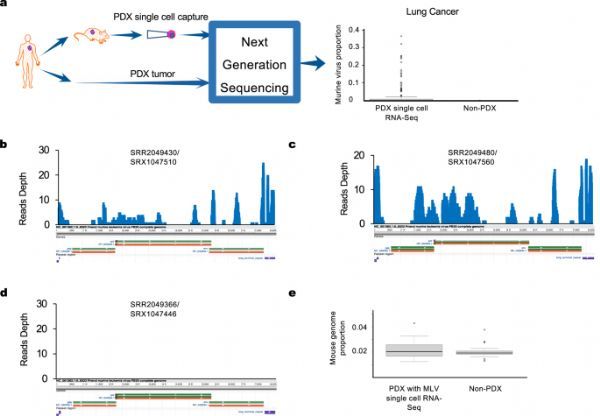
Human-derived tumor xenotransplantation (PDX) is a transplanted tumor model formed by implanting tumor tissues and primary cells from patients into immunodeficient mice. It is often used in the screening of preclinical anti-cancer drugs. The traditional cell line transplantation (CDX) model, PDX model can better maintain the genetic characteristics and heterogeneity of the primary tumor.
According to statistics, as of July 2020, the world’s first free cancer model portal “PDX finder” has saved 4031 PDX models, and the Mouse Tumor Biology Database (MTB) has saved 19,242 and Papers related to the PDX model. Currently, there are 210 NIH-funded PDX projects in progress. It is estimated that by 2022, the total size of the PDX market will reach 167.6 million U.S. dollars, and the total annual budget will exceed 116 million U.S. dollars.
However, what is puzzling is why many anticancer drugs can significantly inhibit the growth of tumors in mice in the PDX model, but they have been repeatedly frustrated in clinical trials?
Recently, researchers from the University of Texas-Houston Health Sciences Center (UTHealth) School of Biomedical Informatics and McGovern School of Medicine jointly published an article entitled “Presence of complete murine viral genome sequences in patient” in the journal Nature Communications. -derived xenografts” article, the study found that the mouse PDX model may be infected by the virus and affect the preclinical drug evaluation, making many anti-cancer drugs that can eliminate tumor cells in the mouse model fail to work in human trials.
First, the researchers sequenced the tumor samples of 7 PDX models based on RNA-Seq technology (170 out of 184 conventional RNA sequence samples), and further verified the test results through fluorescent quantitative PCR technology. Studies have found that mouse PDX tumor cells contain abundant murine virus sequences, and nearly half of the murine viruses are “murine leukemia viruses.”
In order to further confirm the existence of virus sequences in mouse tumor cells, the researchers isolated and sequenced the RNA molecules of individual cells in PDX tumor samples based on single-cell sequencing technology, and found that the murine virus sequence readings in PDX tumor samples were significantly higher than the original Primary tumors, and the murine virus comes from the tissues and blood inside the mouse PDX tumor cells.
So how do these viruses affect the testing of human anti-tumor drug models? Researchers conducted an in-depth analysis of the gene expression of murine viruses and found that the murine viral load of tumor samples was closely related to the expression of immune genes (such as CD80) in the human body. In tumor samples with high viral load, it was associated with cancer, The expression levels of genes related to immunity and drug metabolism also changed significantly.
The traditional concept believes that because the human tumor tissue transplanted into mice has not been processed, most of the characteristics of primary tumors are retained at the histopathology, molecular biology and genetic level, so it has a good clinical effect. Predictive, but maybe this is not the case. The study proposed that although certain anti-cancer drugs have significant effects in animal trials, they may be ineffective in human trials because the drug may kill only cancer cells infected with the mouse virus.
The development of new anti-cancer drugs has a tortuous and long road. In the future, it is still necessary to continue to comprehensively evaluate the impact of PDX models on drug development, strictly control quality, and regularly conduct virus infection tests on animal models to advance the drug development process.
(source:internet, reference only)
Disclaimer of medicaltrend.org



