The world’s first red blood cell maturation agent: Bristol-Myers Reblozyl
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
The world’s first red blood cell maturation agent: Bristol-Myers Reblozyl
The world’s first red blood cell maturation agent: Bristol-Myers Reblozyl treats transfusion-independent β-thalassemia: significantly improves anemia!

Beta thalassemia (picture source: glowydowy.com)
Bristol-Myers Squibb (BMS) and Acceleron Pharmaceuticals recently jointly announced the first results of the Phase 2 BEYOND study. The study is evaluating the first red blood cell maturation agent (EMA) Reblozyl (luspatercept) combined with Best Supportive Care (BSC) for the treatment of non-transfusion dependent (NTD) β-thalassemia (β-thalassemia) adult patients.
The results showed that 77.1% of the patients in the Reblozyl+BSC treatment group achieved increased hemoglobin levels (≥1.0g/dL), compared with 0% in the placebo+BSC group. In addition, compared with the placebo group, patients in the Reblozyl group also reported improved results.
NTD beta thalassemia is a term used to describe patients who do not require regular life-long transfusions of red blood cells (RBC) to maintain survival, although they may require occasional or slightly frequent transfusions, usually within a prescribed period of time.
The investigator of the BEYOND study, Dr. Ali Taher from the American University of Beirut, said: “Patients with transfusion-independent β-thalassemia will experience chronic anemia and iron overload, which may lead to a series of clinical complications and urgent treatment options are needed. Results of the BEYOND study It shows that in adult patients with transfusion-independent β-thalassemia, regardless of the baseline hemoglobin level, Reblozyl has the clinical potential to maintain elevated hemoglobin levels in most patients, while also improving the quality of life of the patients.”
Reblozyl is the first and only red blood cell maturation agent to obtain regulatory approval for the treatment of β-thalassemia, very low/low/medium risk myelodysplastic syndrome (MSD) related anemia. Among the eligible patient groups, Reblozyl represents an important treatment category. It should be pointed out that in patients who need to correct anemia immediately, Reblozyl is not suitable as a substitute for red blood cell transfusion.
The efficacy and safety of Reblozyl in the treatment of β-thalassemia and MDS-related anemia were confirmed in the pivotal Phase 3 BELIEVE and MEDALIST studies, respectively. The BELIEVE study was carried out in patients with transfusion-dependent β-thalassemia, and the MEDALIST study was carried out in patients with very low to median MDS. Both studies met the primary endpoint and all key secondary endpoints. The results showed that compared with the placebo group, the blood transfusion burden of patients in the Reblozyl treatment group was significantly reduced.
The active pharmaceutical ingredient of Reblozyl is luspatercept, which is a first-in-class red blood cell maturation agent (EMA) that can regulate the maturation of late red blood cells. Luspatercept is a soluble fusion protein, which is formed by the fusion of the Fc domain of human IgG1 and the extracellular domain of Activin IIB receptor (ActRIIB). As a ligand trap, it can regulate late RBC maturation through targeted binding. The specific ligand of transforming growth factor (TGF)-β superfamily reduces the activation of Smad2/3 signaling pathway, improves the generation of invalid RBC, promotes the maturation of late RBC red blood cells, and increases hemoglobin levels.
Luspatercept is developed globally by New Base (acquired by BMS) and Acceleron Pharmaceuticals. Currently, both parties are also evaluating the potential of luspatercept to treat erythropoiesis stimulants (ESA) newly treated, low-risk MDS patients (phase III COMMANDS study) and non-transfusion beta thalassemia (phase II BEYOND study) and myelofibrosis.
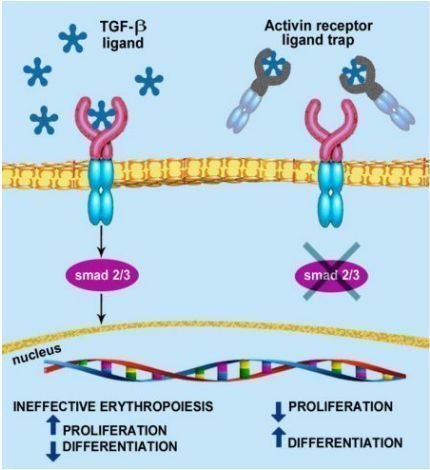
luspatercept mechanism of action
BEYOND is a randomized, double-blind, placebo-controlled, multicenter Phase 2 study in adult patients with transfusion-independent (NTD) β-thalassemia to evaluate the efficacy and safety of Reblozyl relative to placebo. The enrolled patients were: age ≥18 years, suffering from β-thalassemia or hemoglobin (Hb) E β-thalassemia, infused with ≤5 red blood cell (RBC) units within 24 weeks before randomization, with an average baseline Hb ≤10.0 g /Deciliter (g/dL).
In the study, 145 patients were randomized at a 2:1 ratio and received subcutaneous injections of Reblozyl (1 mg/kg [titrated to 1.25 mg/kg]) or placebo every 3 weeks, and the treatment lasted ≥48 weeks. Patients in the 2 groups continued to receive best supportive care (BSC), including RBC infusion and iron chelator treatment. The primary endpoint is: for 12 consecutive weeks starting from week 13-24, the average hemoglobin increased from baseline by 1.0 g/dL without RBC infusion. Secondary endpoints include: the proportion of patients who stayed without infusion from week 1-24, the proportion of patients whose hemoglobin level increased by ≥1.5 g/dL from baseline to week 13-24, and patients with NTD beta thalassemia reported fatigue and weakness (NTDT-PRO T/W) average change in score (the higher the score reflects the worse the quality of life [QoL]).
The results of the primary end point showed that 77.1% (n=74/96) of the patients in the Reblozyl treatment group reached the primary end point, and 0% (n=0/49) in the placebo group. There was a statistically significant difference in the data (p<0.0001). Among patients with an average baseline Hb<8.5g/dL, 72.7% (n=40/55) of the patients in the Reblozyl treatment group reached the primary endpoint, and 0% in the placebo group (p<0.0001); in the average baseline Hb≥8.5 Among patients with g/dL, 82.9% (n=34/41) in the Reblozyl treatment group reached the primary endpoint, and 0% in the placebo group (p<0.0001).
Key secondary endpoints: (1) 52.1% (n=50/96) of the Reblozyl treatment group achieved an average Hb level increase of ≥1.5 g/dL from the baseline between the 13th and 24th week, compared with 0% in the placebo group ( p<0.0001). (2) In week 1-24, 89.6% of patients in the Reblozyl treatment group remained without infusion, and 67.3% in the placebo group (p=0.0013). (3) Improvements in patient-reported quality of life outcomes (fatigue and weakness) are also associated with increased hemoglobin.
In terms of safety, the most common adverse events (TEAEs) that occurred during treatment of any grade in ≥5% of patients were bone pain (Reblozyl group vs placebo group: 36.5% vs 6.1%), headache (30.2% vs 20.4%) ) And joint pain (29.2% vs 14.3%). Among patients treated with Reblozyl, no malignant tumors or thromboembolic events were reported. (
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



