Leukemia (AML): Bristol-Myers Squibb Onureg was approved by EU
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
New drug for Leukemia(AML): Bristol-Myers Squibb Onureg (Azacitidine Tablets) was approved by EU
Leukemia (AML): Bristol-Myers Squibb Onureg was approved by EU. New medicine for leukemia (AML): Bristol-Myers Squibb Onureg (Azacitidine Tablets) approved by EU: the first first-line oral maintenance therapy for patients with AML in remission!

AML (Image source: checkrare.com)
Bristol-Myers Squibb (BMS) recently announced that the European Commission (EC) has approved Onureg (azacitidine tablets) as a first-line oral maintenance therapy for Treatment of acute myeloid leukemia (CR) or complete remission (CRi) with incomplete recovery of platelet count after induction therapy (with or without consolidation therapy), and who are not suitable for or choose not to undergo hematopoietic stem cell transplantation (ASCT) ( AML) adult patients. AML is one of the most common acute leukemias in adults.
It is worth mentioning that Onureg is Europe’s first and only one-time oral first-line maintenance therapy that can be used to treat patients with a wide range of AML subtypes in first remission. After Onureg is listed, it will address the urgent medical needs of the AML patient group for the new maintenance treatment plan. Data from the pivotal QUAZAR AML-001 study showed that in patients with first remission of AML, Onureg first-line maintenance treatment showed significant overall survival (OS) and recurrence-free survival (RFS) benefits compared to placebo. Subgroup analysis showed that OS benefit was consistent in CR or CRi patients.
In the United States, Onureg was approved by the FDA in September 2020 for the continued treatment of adult patients with AML who were in remission for the first time, specifically: for receiving intensive induction chemotherapy to achieve complete remission (CR) for the first time or complete recovery of blood cell counts. Continuing treatment for adult patients with AML who are in remission (CRi) and cannot complete intensive curative therapy (such as ASCT). In the United States, Onureg is the first and only continuation therapy for AML approved by the FDA for patients in remission.
In terms of medication, Onureg can continue until the disease progresses or unacceptable toxicity occurs. Due to the significant differences in pharmacokinetic parameters, Onureg should not replace intravenous or subcutaneous azacitidine.
Onureg’s active pharmaceutical ingredient is CC-486 (azacitidine), which is an oral hypomethylation agent that binds to DNA and RNA, allowing continuous epigenetic regulation due to prolonged exposure. Currently, the drug is being developed as an epigenetic modifier for the treatment of various hematological tumors. The main mechanism of the drug’s action is believed to be DNA hypomethylation and direct cytotoxicity to abnormal hematopoietic cells in the bone marrow. Hypomethylation may restore the normal function of genes essential for differentiation and proliferation.
Onureg-azacitidine chemical structure
AML is the most common type of acute leukemia. AML starts in the bone marrow, but quickly enters the bloodstream. Unlike normal blood cell development, in AML, the rapid accumulation of abnormal white blood cells in the bone marrow may interfere with the production of normal blood cells, resulting in a decrease in healthy white blood cells, red blood cells, and platelets. AML is a complex and diverse disease, which is related to a variety of gene mutations. If left untreated, the condition usually deteriorates rapidly.
Newly diagnosed adult patients with AML can usually achieve complete remission through induction chemotherapy, but many patients will relapse and experience poor results. Patients in remission are in urgent need of a maintenance treatment plan that can reduce the risk of recurrence and prolong overall survival.
This approval is based on the efficacy and safety results of the pivotal Phase 3 QUAZAR AML-001 study. The study was carried out in newly diagnosed AML patients who had achieved first complete remission (CR or CRi) after receiving intensive induction chemotherapy and were not suitable for ASCT at the time of screening. It evaluated the efficacy and safety of Onureg as a first-line maintenance treatment.
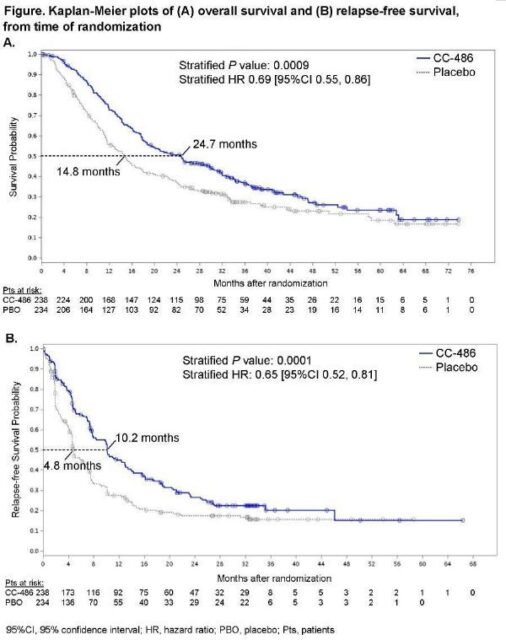
The results showed that compared with placebo, Onureg significantly extended overall survival (OS, primary endpoint) by nearly 10 months in the first-line maintenance treatment (median OS: 24.7 months vs 14.8 months, p=0.0009) , The recurrence-free survival (RFS, key secondary endpoint) was significantly prolonged by more than one time (median PFS: 10.2 months vs 4.8 months, p=0.0001), and the results were statistically significant and clinically significant improvements.
Andrew Wei, the lead investigator of the QUAZAR AML-001 study, said: “In the European Union, although many AML patients get remission through induction therapy, the duration of remission may be short and the risk of recurrence is still high, especially for those who are not eligible for stem cell transplantation. As a result, the demand for AML maintenance treatment options has not been met. The European Commission’s approval of Onureg will enable AML patients to obtain clinical benefits and change the current status of AML treatment, including a series of subtypes.”
QUAZAR AML-001 clinical data
QUAZAR AML-001 is an international, randomized, double-blind, placebo-controlled Phase III study. The enrolled patients were aged ≥55 years, de novo or secondary acute myeloid leukemia, Medium or high risk cytogenetics, first complete remission (CR) or complete remission with incomplete blood recovery (CRi) after intensive induction chemotherapy. According to the investigator’s choice, the patient received intensive induction chemotherapy, with or without consolidation chemotherapy, and was considered not a candidate for hematopoietic stem cell transplantation (HSCT) before the start of the study.
After intensive induction chemotherapy, 81% of patients achieved CR, and 19% of patients achieved CRi. 80% of patients received at least one cycle of consolidation therapy before participating in the study. 472 patients were randomly divided into two groups at a ratio of 1:1, and received: Onureg 300 mg treatment (n=238), placebo treatment (n=234), once a day, each cycle of treatment for 14 days, every 28 Day is a cycle. In the study, patients continue to receive treatment until unacceptable toxicity or disease progression.
With a median follow-up of 41.2 months, the Onureg treatment group showed a significant improvement in the primary endpoint of OS compared with the placebo group. The median OS from the randomization time point in the Onureg treatment group was 24.7 months, while that in the placebo group was 14.8 months (p=0.0009; HR=0.69 [95%CI: 0.55, 0.86]). In terms of key secondary endpoint RFS, the median RFS was 10.2 months in the Onureg treatment group and 4.8 months in the placebo group (p=0.0001; HR=0.65 [95%CI: 0.52, 0.81]).
Regardless of the cytogenetic risk category, previous consolidation status, or CR/CRi status at the time of enrollment, OS and RFS in the Onureg treatment group were improved compared with the placebo group. Compared with the placebo group, the health-related quality of life (HRQoL) in the Onureg treatment group remained unchanged from baseline.
Onureg’s median treatment duration was 12 cycles (1-80), and placebo was 6 cycles (1-73). The most common adverse events (AE) in all grades of Onureg and placebo were nausea (65% vs 24%), vomiting (60% vs 10%), and diarrhea (50% vs 22%). The most common grade 3-4 adverse events of CC-486 and placebo were neutropenia (41% vs 24%), thrombocytopenia (23% vs 22%), and anemia (14% vs 13%). 34% and 25% of the patients in the Onureg treatment group and the placebo group had serious adverse events, mainly infections, which occurred in 17% and 8% of the patients in the two groups, respectively. 13% and 4% of the patients in the Onureg treatment group and the placebo group discontinued treatment due to AEs.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



