BiTE immunotherapy: Amgen Blincyto treats acute lymphoblastic leukemia
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
BiTE immunotherapy: Amgen Blincyto treats acute lymphoblastic leukemia
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- What is the difference between Atorvastatin and Rosuvastatin?
- How long can the patient live after heart stent surgery?
BiTE immunotherapy: Amgen Blincyto treats acute lymphoblastic leukemia.
Amgen Blincyto has a significant clinical effect in the treatment of pediatric patients with acute lymphoblastic leukemia (ALL)!

Amgen recently announced that data from a multicenter randomized phase 3 clinical study (20120215, NCT02393859) evaluating BiTE immunotherapy Blincyto (blinatumomab) in the treatment of pediatric patients with acute lymphoblastic leukemia (ALL) has been published in the American Medical Association Magazine (JAMA).
In addition, the results of a risk stratification randomized phase 3 clinical study (COG AALL1331) evaluating Blincyto for the treatment of first recurrence in pediatric B-ALL patients were also published in JAMA.
In these two studies, Blincyto treatment was less toxic, and minimal residual disease (MRD) negative remission rate was higher.
Blincyto is the first and only CD19-CD3 bispecific T cell junction (BiTE) immunotherapy approved globally.
It is also the first bispecific antibody product born on the Amgen BiTE technology platform. The CD19 protein is presented to the CD3 protein specifically expressed by T cells, which then activates the immune system to recognize and kill tumor cells.
David M. Reese, Executive Vice President of Amgen Research and Development, said: “Acute lymphocytic leukemia (ALL) is the most common type of cancer in children.
Unfortunately, about 15% of high-risk B-ALL children relapse after first-line chemotherapy.
These patients There is still an urgent need for new treatment options. The above research results support Blincyto as a new standard therapy for the consolidation of patients with this aggressive disease.”
The 20120215 study is an open-label, randomized, controlled, global multi-center phase 3 study, carried out in high-risk, first relapsed B-cell precursor acute lymphoblastic leukemia (B-ALL) pediatric patients, and compared the differences in allogeneic hematopoiesis The efficacy, safety, and tolerability of Blincyto or consolidation chemotherapy before stem cell transplantation (alloHSCT).
In September 2019, due to the encouraging efficacy observed in the Blincyto treatment group, according to the recommendation of the Independent Data Monitoring Committee (DMC), the enrollment of patients in the study was terminated early. The follow-up will continue to advance according to the clinical plan.
The results published in JAMA showed that compared with chemotherapy, Blincyto showed a significant prolongation of event-free survival (events are defined as: recurrence, death, second malignancy, failure to achieve complete remission).
After a median follow-up of 22.4 months, 69% of patients in the Blincyto treatment group were alive without incidents, and 43% in the chemotherapy group.
In addition, in patients with minimal residual disease (MRD) at baseline, 93% of patients achieved MRD-negative remission after Blincyto treatment, compared with 24% in the chemotherapy group.
The 36-month overall survival rate (OS) is estimated to be 81.1% in the Blincyto treatment group and 55.8% in the chemotherapy group. The median OS has not yet been reached.
In this study, the incidence of serious adverse events (AE) in the Blincyto group and the chemotherapy group were 24.1% and 43.1%, respectively, and the incidence of grade ≥3 adverse events was 57.4% and 82.4%, respectively.
No fatal adverse events were reported. The most common adverse reactions (AE) in the Blincyto treatment group were fever (81.5%), nausea (40.7%), headache (35.2%), stomatitis (35.2%) and vomiting (29.6%).
The investigator of the study, Franco Locatelli, a researcher at the Department of Pediatric Hematology and Oncology at the Bambino Gesu Children’s Hospital of the University of Rome, said: “The results of the study show that Blincyto is more effective and less toxic than intensive chemotherapy. I am very excited about this.
Before receiving stem cell transplantation, chemotherapy has been used as the main consolidation treatment for all patients, although this method is only partially effective and is related to related toxicity.
Blincyto has now been shown to be used in high-risk B-ALL children with first relapse A more effective and safer consolidation treatment program.”
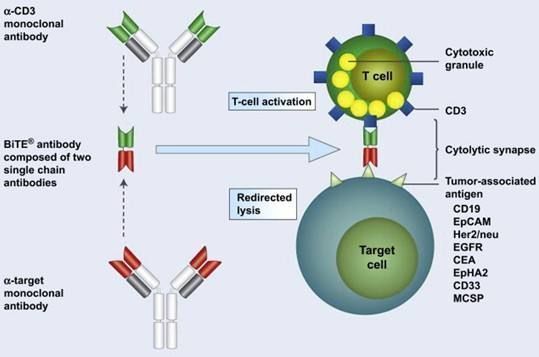 BiTE immunotherapy mechanism of action
BiTE immunotherapy mechanism of action
BiTE antibody technology represents an innovative immunotherapy method that can work at very low concentrations.
Amgen acquired BiTE technology after acquiring Micromet in 2012 for US$1.2 billion. Currently, Amgen is exploring the potential of BiTE innovative therapies in a wide range of refractory tumor types.
Blincyto is the first bispecific antibody product born on Amgen’s BiTE technology platform. Previously, the US FDA has granted Blincyto the Orphan Drug Designation (ODD), Breakthrough Drug Designation (BTD), and priority review qualifications for the treatment of various types of hematological cancers, including acute lymphocytic leukemia (ALL), chronic lymphocytic leukemia (CLL), Hairy cell leukemia (HCL), young lymphocytic leukemia (PLL) and indolent B-cell lymphoma, mantle cell leukemia (MCL), etc.
In the United States, Blincyto has been approved for the treatment of:
(1) adult and pediatric patients with relapsed or refractory B-cell precursor ALL;
(2) minimal residual disease (MRD) ≥0.1 in the first or second complete remission % Of B-cell precursor ALL in adults and children.
In the European Union, Blincyto has been approved for the treatment of:
(1) Philadelphia chromosome-negative, CD19-positive, relapsed or refractory acute lymphoblastic leukemia (ALL) adult patients;
(2) minor in the first or second complete remission Residual disease (MRD) ≥0.1% of Philadelphia chromosome-negative, CD19-positive B-cell precursor ALL adult patients;
(3) Relapsed or refractory after receiving at least 2 previous therapies, or after receiving allogeneic hematopoietic stem cell transplantation Relapsed Philadelphia chromosome-negative, CD19-positive B-cell precursor pediatric patient (≥1 year old).
In ALL patients, the detection of residual cancer cells (ie, MRD) after complete remission is the strongest prognostic factor for assessing recurrence.
It is worth mentioning that in the United States and the European Union, Blincyto is the first therapy to obtain regulatory approval to eradicate MRD.
BiTE immunotherapy: Amgen Blincyto treats acute lymphoblastic leukemia
(source:internet, reference only)
Disclaimer of medicaltrend.org



