Lung cancer: Cimeprizumab has a powerful single-agent treatment effect
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Lung cancer: Cimeprizumab (Drug C) has a powerful single-agent treatment effect
Lung cancer: Cimeprizumab (Drug C) has a powerful single-agent treatment effect. The EMPOWER-Lung 1 study is an open-label, randomized, multicenter phase III trial.
Based on the results of Keynote-024 and IMpower110, the FDA has approved pembrolizumab and atezolizumab as the first-line treatment for patients with advanced NSCLC with PD-L1≥50%.
At this year’s World Lung Cancer Conference, the EMPOWER-Lung1 research data was announced and simultaneously published in the journal Lancet, bringing new treatment options for first-line patients with PD-L1 ≥ 50% of advanced NSCLC receiving immune checkpoint inhibitors.

Research method
The EMPOWER-Lung 1 study is an open-label, randomized, multi-center phase III trial that included 710 patients with locally advanced phase IIIB/C that were not suitable for surgical resection or existing radiotherapy and chemotherapy regimens, and advanced after receiving existing radiotherapy and chemotherapy regimens NSCLC patients, or previously untreated metastatic stage IV NSCLC patients;
All patients have high expression of PD-L1 (≥50%);
Patients with well-controlled hepatitis B/C/HIV, pre-treated and stable brain metastases, squamous and non-squamous NSCLC can participate in the trial.
In the original trial design, all patients were randomly divided into two groups.
The patients were randomized to receive cimiprizumab 350 mg intravenously at a ratio of 1:1, once every 3 weeks for 108 weeks; or the platinum-containing dual-drug chemotherapy regimen selected by the investigator for 4-6 cycles (with Or without pemetrexed maintenance chemotherapy).
If the condition of any group of patients deteriorates severely, cross-group treatment is allowed. The primary end point is the patient’s overall survival (OS), and the secondary end points include objective response rate (ORR), duration of remission, quality of life, and safety.
Research results
From June 2017 to February 2020, a total of 710 patients were randomized (356 cases in the cimipril group and 354 cases in the chemotherapy group). In the second interim analysis in March 2020, 355 and 342 patients in the overall population received cimiprizumab and chemotherapy, respectively.
Among patients with disease progression in the chemotherapy group, 73.9% (150/203) received cimiprizumab cross-treatment; among patients with disease progression in the cimiprizumab group, 31.6% (50/158) were Chemotherapy is added to the treatment of cimiprizumab.
In the intent-to-treat (ITT) population with PD-L1≥50%, 283 and 280 patients received cimiprizumab and chemotherapy, respectively. Among them, 88 cases did not follow the entry criteria for PD-L1 testing before August 2018, and showed PD-L1 ≥50% when tested again; 475 cases followed the entry criteria for PD-L1 testing after August 2018 and showed PD -L1≥50%.
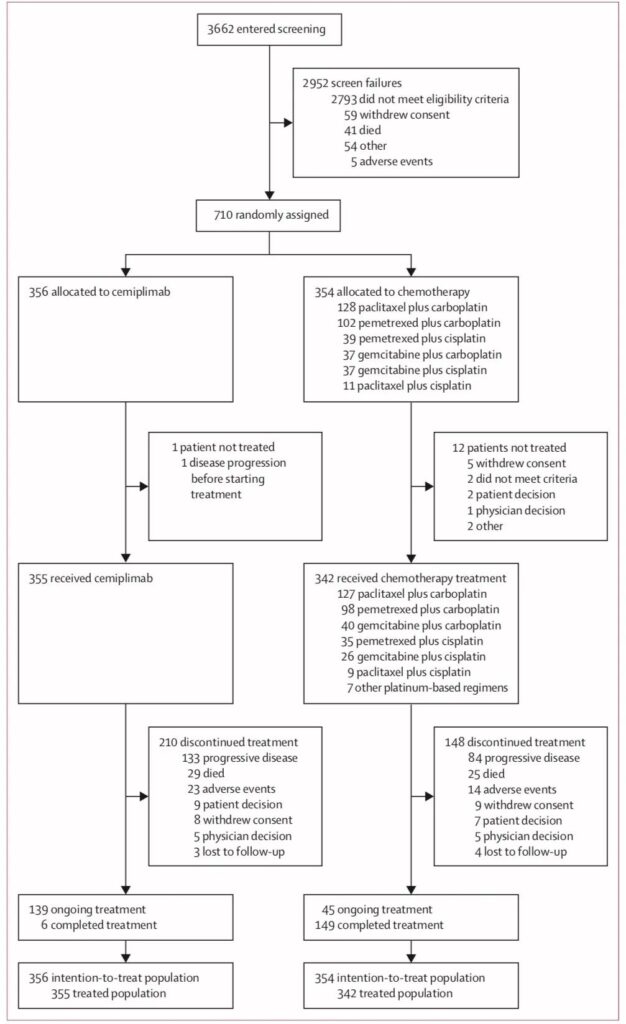
Research design
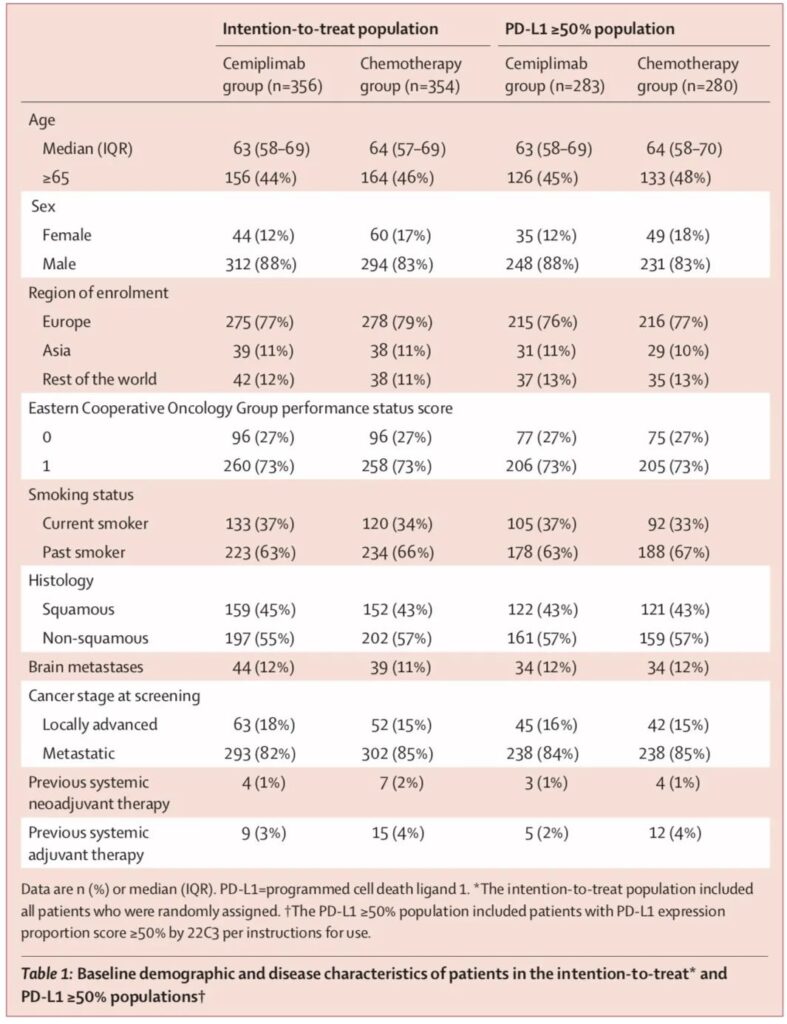
Patient baseline characteristics
Intention-to-treat (ITT) population:
The ITT population (n = 710) consisted of 563 patients with PD-L1>50% and 56 patients with clear PD-L1 expression below 50%.
The median follow-up time for cimiprizumab and chemotherapy was 13.1 months, respectively. In the ITT population, compared with chemotherapy, cimiprizumab improved in OS, respectively 22.1 months (95% CI, 17.7, NE) and 14.3 months (95% CI, 11.7-19.2) (HR, 0.68; 95% CI, 0.53-0.87; P = .0022).
Cimiprizumab also improved PFS compared to chemotherapy. The median PFS was 6.2 months (95% CI, 4.5-8.3) and 5.6 months (95% CI, 4.5-6.1) (HR, 0.59; 95%) CI, 0.49-0.72; P <.0001).
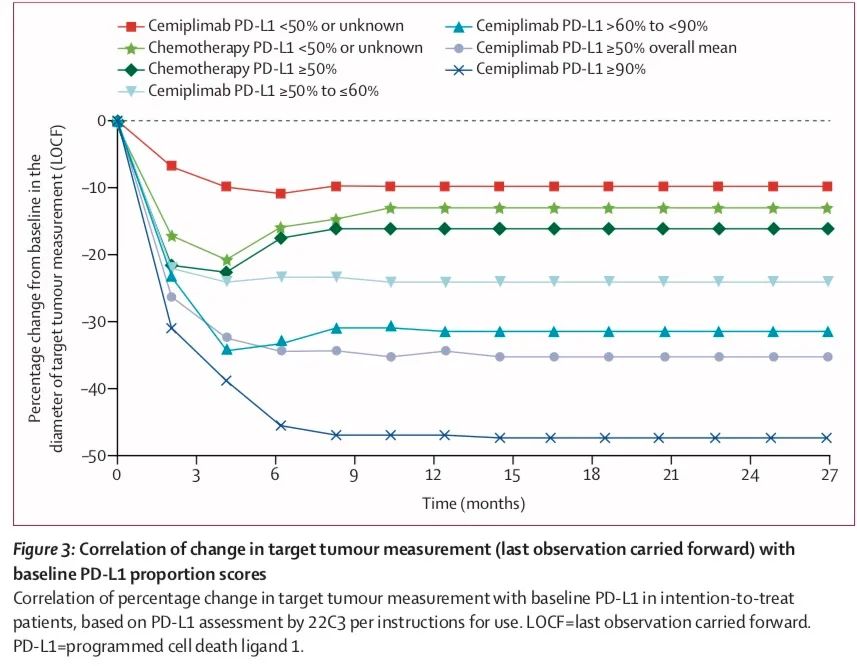
ITT crowd
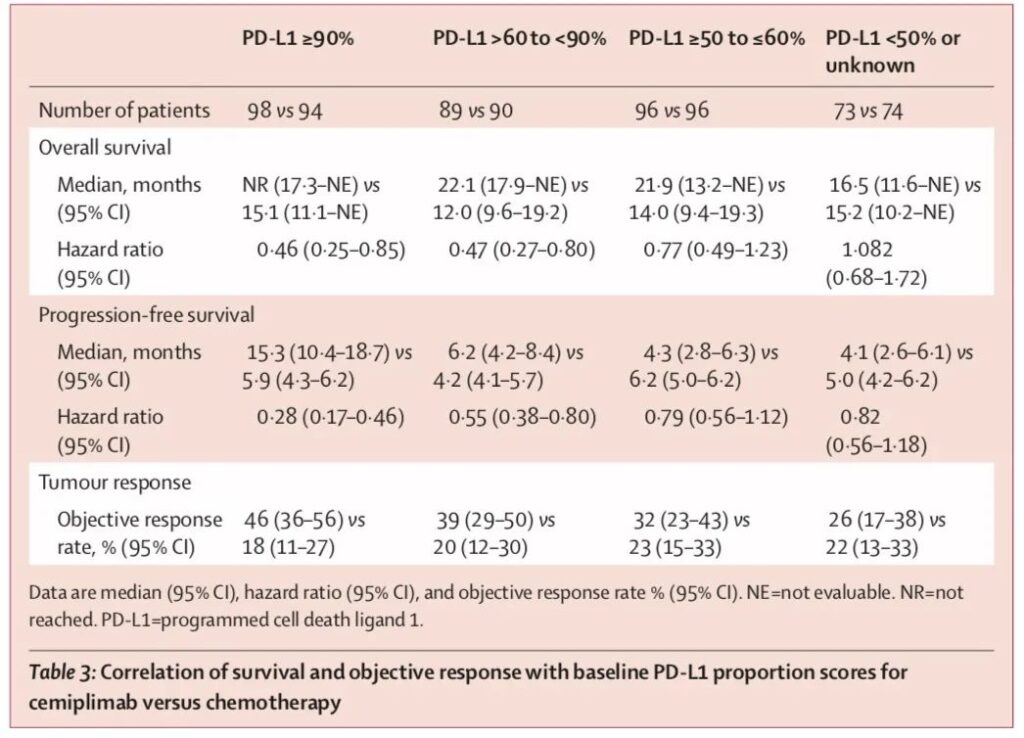
PD-L1≥50% of ITT population
ITT population with PD-L1≥50%:
In the ITT population with PD-L1≥50%, the median follow-up time of the cimiprizumab group and the chemotherapy group were 10.8 and 10.2 months, respectively, and 111 of the 283 patients who received cimiprizumab (39%; 95% CI 34-45) and 57 of the 280 patients receiving chemotherapy (20%; 16-26) observed the objective response assessed by IRC. The median period of remission (DoR) was 16.7 vs 6.0 months, respectively.
The median OS of cimiprizumab has not yet reached the median value (95% CI, 17.9-NE), while chemotherapy is 14.2 months (95% CI, 11.2-17.5) (HR 0.57; 95% CI 0.42-0.77; P = .0002).
In addition, the estimated rates of 24-month OS in the two groups were 50% and 27%, respectively.
Exploratory analysis:
Exploratory analysis of PD-L1 expression level (PD-L1≥90%; PD-L1>60% to <90%; PD-L1≥50% to ≤60%):
1) Compared with the chemotherapy group, the PD-1 group has better survival (OS), progression-free survival (PFS) and objective response rate (ORR), and the extent of benefit increases with the increase of PD-L1 expression rise;
2) The tumor shrinkage in the study group was greater, and it was related to the PD-L1 level;
3) In the PD-1 monoclonal antibody group and the chemotherapy group, the ORR difference of the population with PD-L1≥90% is the largest: 46% vs 18%;
4) PD-L1<50% or unknown, the curative effect is roughly the same as that of patients receiving chemotherapy.
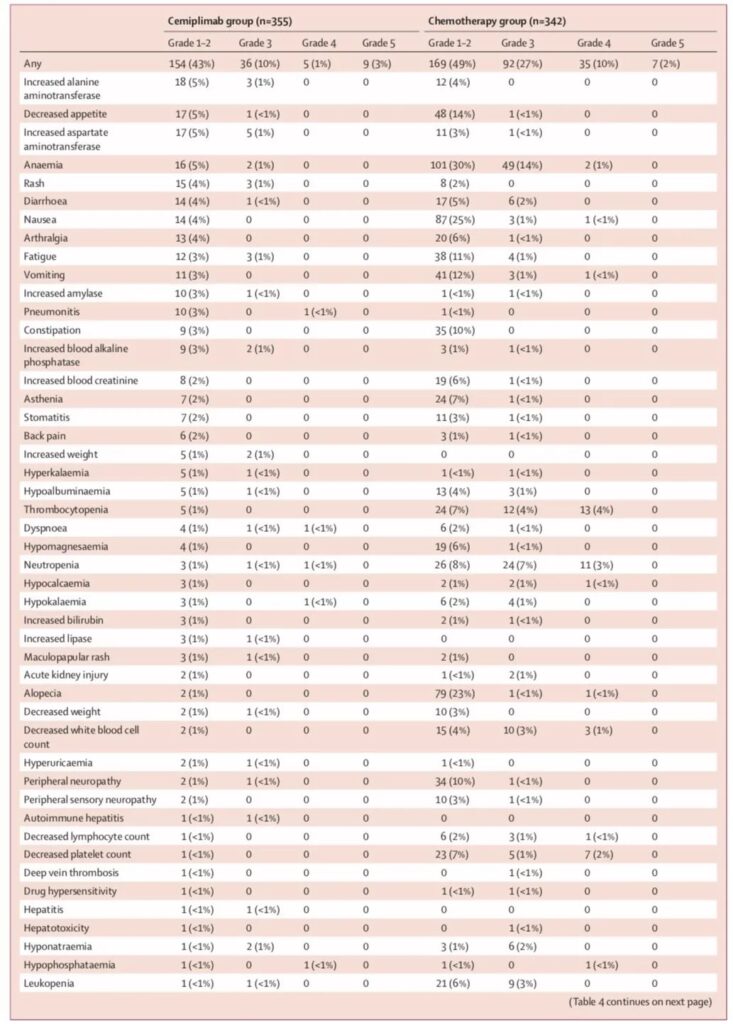
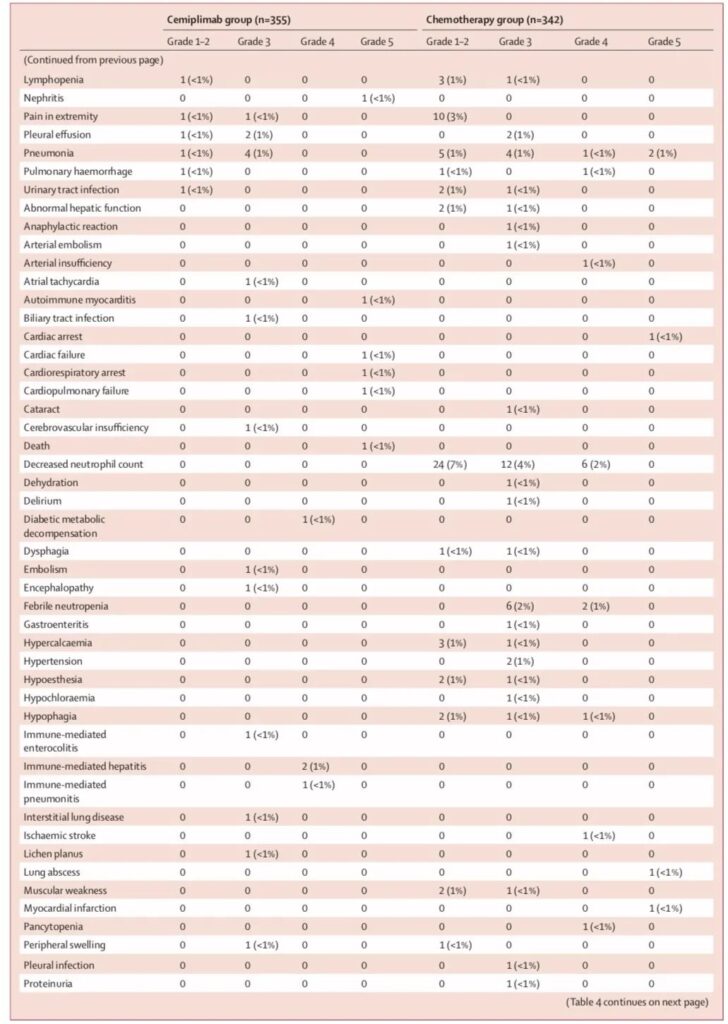
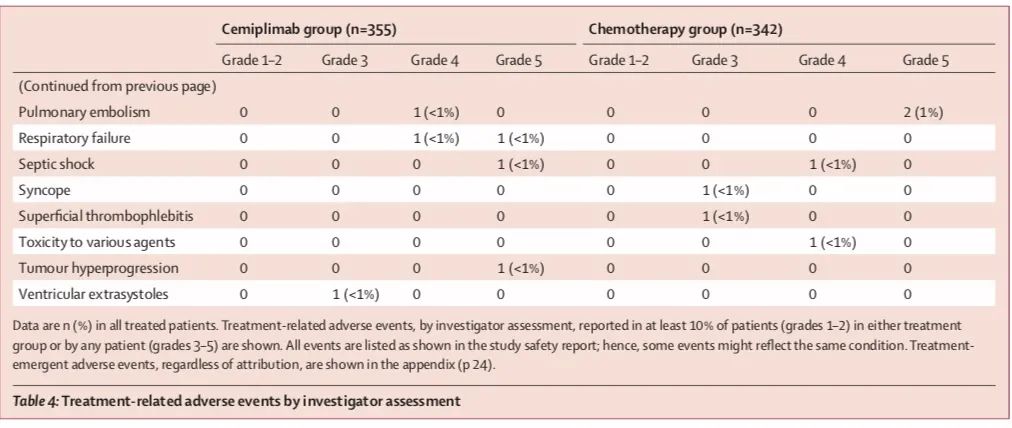
Safety:
In the cimiprizumab group, 28% (98/355) of patients had grade 3-4 adverse events, of which 16 cases (5%) of pneumonia, 12 cases of anemia (3%), and hyponatremia 9 Cases (3%); 39% (135/342) in the chemotherapy group, of which 56 cases (16%) were the most common with anemia, and 35 cases (10%) with neutropenia.
Based on the above research results, on February 22, 2021, the U.S. Food and Drug Administration (FDA) approved the PD-1 inhibitor cimiprizumab as the first-line treatment of PD-L1 expression ≥50%, without EGFR, ALK or Patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) who have ROS1 mutations and are not suitable for surgical resection or radiotherapy and chemotherapy.
(source:internet, reference only)
Disclaimer of medicaltrend.org



