Endoplasmic reticulum stress signal in tumor and its microenvironment
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Endoplasmic reticulum stress signal in tumor and its microenvironment
Endoplasmic reticulum stress signal in tumor and its microenvironment. The endoplasmic reticulum (ER) is the central organelle for secreted and transmembrane protein synthesis, folding and modification.
Protein processing, modification and folding in ER are strict regulatory processes that determine cell function, fate and survival. In several tumor types, different carcinogenic, transcriptional and metabolic abnormalities work together to create an unfavorable microenvironment, destroy the endoplasmic reticulum homeostasis of tumor cells and mesenchymal cells, and infiltrate lymphocytes.
These changes trigger a continuous endoplasmic reticulum stress state, which has been shown to control a variety of tumor-promoting properties of cancer cells, while dynamically reprogramming the functions of innate immune cells and adaptive immune cells.
The abnormal activation of the ER stress sensor and its downstream signaling pathways has become a key regulator of tumor growth and metastasis, as well as the response to chemotherapy, targeted therapy and immunotherapy.
Common drivers of ER stress in TME
The folding and modification of endoplasmic reticulum proteins is a highly regulated process. However, various endogenous and exogenous stresses can destroy protein homeostasis in organelles, leading to endoplasmic reticulum stress.
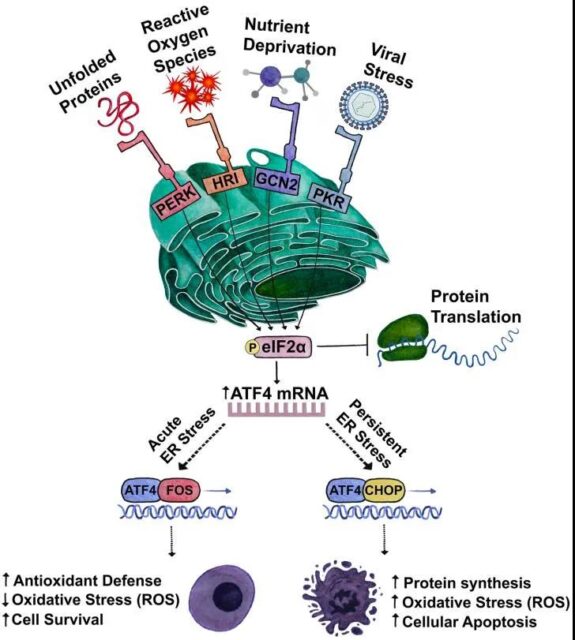
The unfolded protein response (UPR) is a highly conservative adaptive mechanism with three branches that coordinate the response to the harmful accumulation of unfolded or misfolded proteins. They include: inositol enzyme 1α (IRE1α), PRKR-like ER kinase (PERK) and activated transcription factor 6 (AFT6).
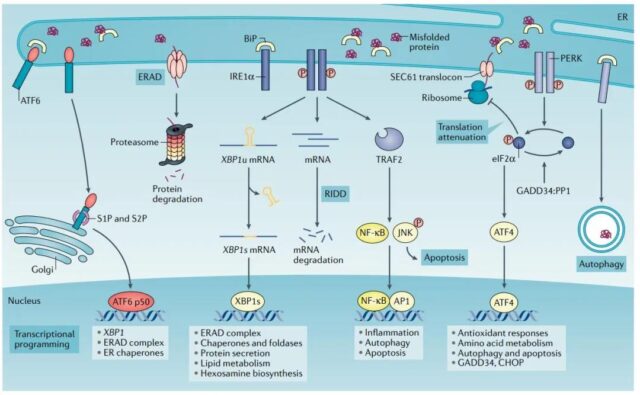
The abundant multiple stressors in TME dynamically interfere with the protein folding ability of the endoplasmic reticulum of malignant cells and mesenchymal cells.
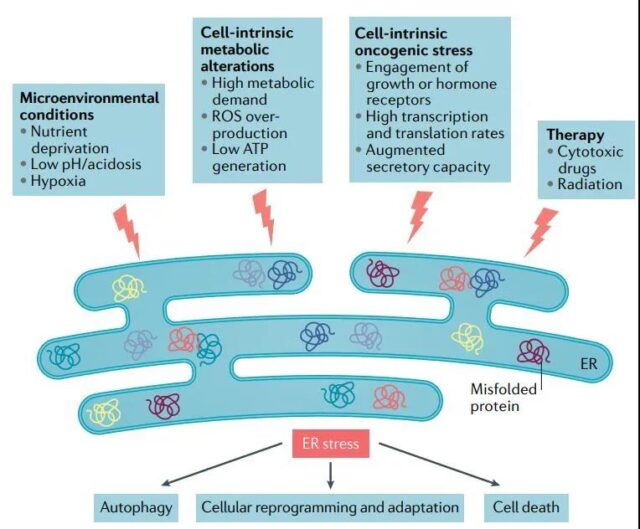
Hypoxia: Hypoxia is a common feature of TME, which disrupts the homeostasis of the endoplasmic reticulum. The formation of disulfide bonds during protein synthesis can occur in the absence of oxygen, but post-translational folding or isomerization is an oxygen-dependent process. In addition, hypoxia limits the function of the oxygen-dependent endoplasmic reticulum local oxidoreductase ERO1α, which is required for the formation of disulfide bonds and protein folding.
Nutrition: Compared with normal energy requirements, metabolic stress is characterized by insufficient or excessive nutrient supply, which can easily destroy the homeostasis of the endoplasmic reticulum. The effectiveness of glucose and glutamine is closely related to endoplasmic reticulum stress.
Reactive oxygen species: The folding of proteins in the endoplasmic reticulum largely depends on the redox state of this organelle. The accumulation of reactive oxygen species (ROS) in cells in response to external conditions or caused by different signal events can greatly disrupt the expression of endoplasmic reticulum proteins.
Low pH: Cancer cells use aerobic glycolysis as a central metabolic pathway to produce lactic acid and lower the pH of the surrounding microenvironment.
Endoplasmic reticulum stress response of tumor cells
The role of UPR in tumor transformation and tumor growth
Carcinogenic transformation is a multi-step process that uses UPR to overcome various obstacles. Excessive activation of MYC in normal epithelial cells will produce a large amount of protein toxic stress and lead to a decrease in cell survival rate. However, in a variety of human cancers including lymphoma, neuroblastoma, prostate cancer, and breast cancer, cells subjected to MYC-induced stress show enhanced UPR activity. Therefore, a fully activated UPR is essential to adapt to the stress caused by MYC-driven oncogene transformation.
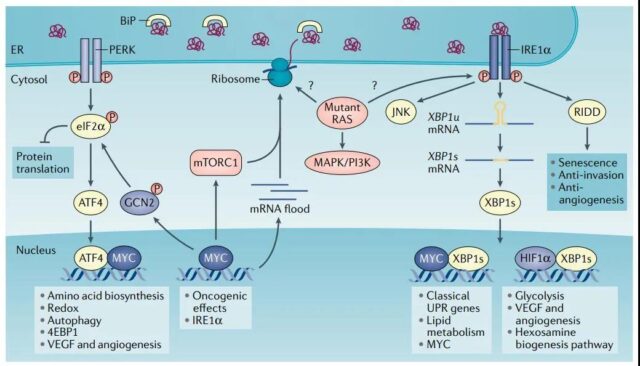
Mutant RAS is another oncogenic driver that interacts with UPR. Acquired drug resistance allows KRAS mutant lung cancer cells to bypass the typical KRAS effector, but requires overactive AXL/eIF4E to increase protein turnover in the ER, and ER stress relieves the adaptive activation of the UPR survival pathway, and HSP90 is maintained. Its completeness. HSP90 inhibitors can synergistically enhance the anti-tumor effects of MTA and trametinib.
Molecular chaperone binding immunoglobulin (BiP) is overexpressed in a variety of human cancers, and promotes tumor growth through a variety of mechanisms, such as promoting the maturation and secretion of growth factors, inhibiting cell apoptosis and promoting angiogenesis. Therefore, BiP is considered to be an attractive target for the treatment of human cancer.
PERK uses various mechanisms to regulate tumor progression. First, one of its key functions is to control oxidative stress by increasing the biosynthesis of the antioxidant glutathione. Second, the PERK-eIF2α axis attenuates overall transcription and enhances autophagy to promote the cytoprotective UPR function in MYC-driven lymphoma. Third, both PERK and GCN2 are activated by MYC to phosphorylate eIF2α and induce ATF4.
The role of UPR in transfer and hibernation
Some studies have shown that the PERK-eIF2α axis inhibits anoikis, which is necessary for tumor invasion and metastasis. PERK is also selectively activated in cells undergoing epithelial-mesenchymal transition (EMT), and its secretion capacity is enhanced. In primary tumors, the level of oxidative stress in circulating and distant tissues of metastatic cells is higher than that of cancer cells. Metabolic adaptation, such as the synthesis of antioxidants, is essential for the survival and ultimate growth of cancer cells in the distance. The PERK branch promotes the antioxidant response through ATF4 and NRF2, and therefore, may benefit metastatic cells by reducing oxidative stress.
Recent studies have also shown an increase in the UPR response of dormant malignant cells from patients and mouse cancer models, including breast, squamous, colorectal, and pancreatic ductal adenocarcinoma (PDAC). UPR may induce dormancy as an adaptation for survival in the unfavorable microenvironment of remote organs.
Regulation of tumor cell ER stress on tumor immune microenvironment
A large number of studies have shown that the internal ER stress response of tumor cells can affect tumor progression by changing the function of immune cells coexisting in TME.
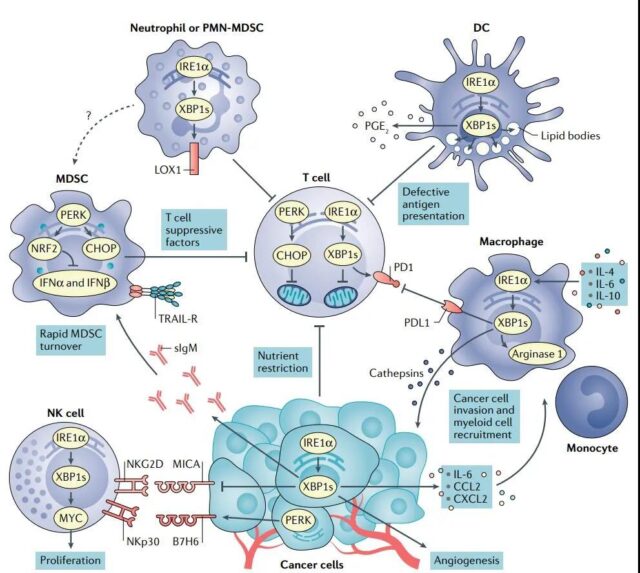
Early studies have shown that the induction of ER stress and the activation of UPR may inhibit the surface expression of major histocompatibility complex class I (MHC-I) molecules through the overexpression of XBP1s and ATF6.
The ER stress response of tumor cells is thought to change the natural killer cell (NK)-mediated tumor recognition. Studies have shown that in melanoma cells undergoing drug-induced ER stress, PERK–eIF2α axis activation of UPR can induce the expression of B7H6, which is the ligand of NK cell receptor NKp30.
ER-stressed tumor cells can release additional factors to recruit or change the function of myeloid cells in the tumor.
ER stress of tumor cells can also regulate T cell-mediated tumor growth, metastasis and response to immunotherapy. In a cohort of various melanoma patients receiving anti-CTLA-4 treatment, the decreased expression of XBP1s, ATF4 and BiP was associated with improved efficacy and prolonged survival.
UPR of immune cells in tumor
The high metabolic demand and unlimited proliferation of tumor cells have greatly changed the nutritional composition of the tumor environment, while tumor-infiltrating immune cells have limited access to key nutrients required for protein folding and effective anti-cancer response. Therefore, tumor-infiltrating lymphocytes continue to activate the ER stress response, in addition to triggering the typical UPR, but also in the immune cell-specific way to regulate the main transcription and metabolic programs.
Targeting the endoplasmic reticulum stress sensor or its related UPR response pathway may help enhance the effect of immune checkpoint blockade and adoptive T cell immunotherapy on solid tumors that are currently difficult to treat with these methods.
Drug research targeting UPR
The method of combining standard therapies with UPR modulators has shown significant efficacy in preclinical cancer models, so it is worthy of further research in cancer patients.
IRE1α inhibitor: IRE1α has two targetable enzyme domains: kinase domain and endorphinase domain. IRE1α kinase inhibitors have shown significant in vivo efficacy in multiple myeloma xenograft models. The IRE1α kinase inhibitor KIRA8 or AMG-18 inhibits the growth of multiple myeloma and enhances the response of these tumors to established first-line drugs, the proteasome inhibitor bortezomib and the immunomodulatory drug lenalidomide.
IRE1αRNase inhibitors, including B-I09, STF083010, MKC3946 and MKC8866, have been extensively tested in mouse models of breast cancer, prostate cancer, melanoma, lymphoma, multiple myeloma and CLL. B-I09 has been proven to be a safe and selective IRE1αRNase inhibitor, suitable for use in vivo. B-I09 inhibits the growth of leukemia in CLL mouse models without causing systemic toxicity, and synergizes with the FDA-approved Bruton tyrosine kinase (BTK) inhibitor ibrutinib to induce B-cell leukemia, lymphoma and multiple myeloma Apoptosis of human cell lines.
PERK inhibitors: PERK inhibitors GSK2606414 and GSK2656157 inhibit tumor growth in human xenograft models of different cancers. GSK2656157 can also make colon cancer cells sensitive to 5-fluorouracil (5-FU) chemotherapy drugs. Interestingly, in a mouse model of immunogenic sarcoma, GSK2606414 can activate T cell function and enhance the response to PD-1 blockade. Despite its remarkable curative effect, PERK inhibition has serious toxic effects on the pancreas and significantly inhibits the production of insulin.
Based on structure design and optimization, the compounds AMG44 and AMG52 are considered to be effective and highly selective PERK inhibitors. These two compounds have good pharmacokinetic properties and tolerability in vivo, and further preclinical and clinical studies are needed to evaluate their anti-tumor efficacy and potential side effects in combination with cytotoxic drugs or targeted therapies.
eIF2α inhibitor: ISRIB is a potent eIF2α inhibitor that inhibits the phosphorylation of eIF2α by activating eIF2B. When used as a single drug, ISRIB can significantly inhibit the progression of prostate cancer with PTEN deficiency and MYC overexpression, and prolong the survival of tumor-bearing mice. Importantly, ISRIB alone can lead to regression of advanced prostate tumors after 3 weeks of treatment without obvious side effects.
BiP inhibitor: In various mouse tumor models, the BiP inhibitor KP1339 induces a wide range of ER stress and immunogenic cell death. The phase I study of KP1339 showed that 10 of 38 patients with metastatic neuroendocrine tumors, non-small cell lung cancer (NSCLC) or colon cancer had stable disease and acceptable tolerance.
HA15 is another compound targeting BiP. In a xenograft model of melanoma, HA15 induces a significant anti-tumor effect and effectively slows down BRAF inhibitor-resistant melanoma without major side effects.
Outlook
Persistent endoplasmic reticulum stress is a new feature of tumors. It is caused by various metabolic and carcinogenic abnormal factors in TME. These abnormalities disturb the protein folding homeostasis of tumor cells and infiltrating immune cells. The active endoplasmic reticulum stress response allows tumor cells to adapt to carcinogenic and environmental challenges, while coordinating different immune regulatory mechanisms to promote tumor progression.
Targeting the endoplasmic reticulum stress response can enhance anti-tumor immunity while destroying certain aggressive characteristics of tumor cells. More and more evidence shows that the regulation of the endoplasmic reticulum stress sensor or UPR response will make aggressive tumors more sensitive to cytotoxic drugs, targeted therapy and immunotherapy. Larger preclinical studies and retrospective analysis of clinical trial samples will help to discover effective UPR targeted combination therapies to obtain a durable response that prevents cancer progression and/or recurrence.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



