FDA: Radioligand therapy as “breakthrough therapy” for prostate cancer
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
FDA: Radioligand therapy as “breakthrough therapy” for prostate cancer
FDA: Radioligand therapy as “breakthrough therapy” for prostate cancer. Recently, the US FDA granted the radioligand therapy 177Lu-PSMA-617 “Breakthrough Therapy” designation for the treatment of prostate-specific membrane antigen (PSMA) positive castration-resistant prostate cancer (mCRPC) patients.
The results announced at the 2021 ASCO annual meeting showed: 177Lu-PSMA-617 significantly improved the overall survival and radiological progression-free survival of patients, reaching the dual primary endpoint of the trial.
▌The second most common cancer in men: prostate cancer
Prostate cancer is the second most common type of cancer in men. It is estimated that there are approximately 1.3 million newly diagnosed cases worldwide each year, of which approximately 100,000 are newly diagnosed in China each year.
At present, the treatment of choice for patients who are not suitable for radical resection is androgen deprivation therapy (ADT), which includes GnRH agonist and antagonist drug therapy. This therapy reduces testosterone to very low levels, commonly referred to as castration levels.
And metastatic castration-resistant prostate cancer (mCRPC) is the final stage of the development of prostate cancer disease, and the response to hormone therapy is insufficient. About 10-20% of advanced patients will develop CRPC within 5 years. The 5-year survival rate is low and the mortality rate is high.
Although progress has been made in the treatment of mCRPC in recent years, the 5-year survival rate of patients is low, less than 17%, there are unmet medical needs, and new treatment options are urgently needed.
▌Targeted radioligand therapy: 177Lu-PSMA-617
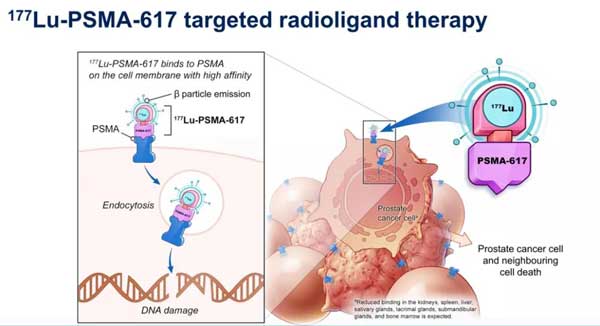
The radioligand therapy 177Lu-PSMA-617 can be combined with targeting prostate specific membrane antigen (PSMA), which is a specific molecular marker for prostate cancer. It is highly expressed in more than 80% of prostate cancers and is a potential Therapeutic target.
177Lu-PSMA-617 is composed of a therapeutic radioisotope and a ligand targeting PSMA. It can deliver the radioisotope-carrying drug into the patient’s body and combine with PSMA to locally release radiation energy to kill tumor cells. Because the radiation works locally, it greatly limits the damage of the drug to the surrounding healthy cells and reduces the side effects of radiotherapy.
▌Clinical trials
This identification is based on positive data from the ongoing randomized double-blind, open-label, multi-center Phase 3 VISION study.
The trial enrolled a total of 831 patients, randomly assigned at a ratio of 2:1, and aimed to study the efficacy of 177Lu-PSMA-617 compared with standard therapy (SOC) in patients with progressive PSMA-positive mCRPC. The primary endpoints are OS and rPFS.
The research results announced at the 2021 American Society of Clinical Oncology (ASCO) annual meeting show that:
·Compared with standard therapy, 177Lu-PSMA-617 can reduce the patient’s risk of death by about 40%. The noon overall survival of patients in the treatment group was extended by 4 months to 15.3 months, while the OS of the control group was 11.3 months.
· In addition, 177Lu-PSMA-617 also improved radiological progression-free survival by 5.3 months, which is equivalent to a 60% reduction in the risk of progression or death. The median rPFS of the treatment group and the control group were 8.7 months and 3.4 months, respectively.
· In terms of secondary endpoints, the objective remission rates of the treatment group and the control group were 29.8% and 1.7%, respectively; the disease control rates were 89% and 66.7%, respectively.
Currently, the test is still in progress. Two studies of radioligand therapy in the early treatment of metastatic prostate cancer are ongoing, focusing on the potential clinical utility of this therapy in the mCRPC taxane pre-environment and metastatic hormone-sensitive environment.
The targeted radioligand therapy 177Lu-PSMA-617 can directly target prostate cancer cells, allowing us to see a precise radiotherapy plan that replaces traditional therapies. The therapy is expected to submit a marketing application in the second half of this year, bringing a new treatment option for patients with metastatic prostate cancer.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



