NEJM: mRNA and adenovirus vector vaccine effectively prevents Delta variant
- Why Botulinum Toxin Reigns as One of the Deadliest Poisons?
- FDA Approves Pfizer’s One-Time Gene Therapy for Hemophilia B: $3.5 Million per Dose
- Aspirin: Study Finds Greater Benefits for These Colorectal Cancer Patients
- Cancer Can Occur Without Genetic Mutations?
- Statins Lower Blood Lipids: How Long is a Course?
- Warning: Smartwatch Blood Sugar Measurement Deemed Dangerous
NEJM: mRNA and adenovirus vector COVID-19 vaccine effectively prevents Delta variant
NEJM: mRNA and adenovirus vector vaccine effectively prevents Delta variant. From the perspective of clinical treatment, the protective effect of vaccines is reflected in the prevention of severe diseases and deaths. However, from a public health perspective, reducing the infection rate is equally important.
With the continuous emergence of COVID-19 virus variants with higher transmission and even more pathogenicity, it is increasingly important that various COVID-19 vaccines designed based on wild strains can effectively resist these variants.
On July 22, 2021, the New England Journal of Medicine (NEJM) published online the results of a real-world study in the United Kingdom, showing that the mRNA vaccine BNT162b2 and the adenovirus vector vaccine ChAdOx1 nCoV-19 can effectively prevent the Delta mutant strain first discovered in India ( Also known as B.1.617.2) symptomatic infection caused by.
The study showed that the protective efficacy of two doses of BNT162b2 vaccine against delta variant strains was 88.0%; the protective efficacy of ChAdOx1 nCoV-19 vaccine was relatively poor, at 67.0%. The phase 3 clinical trials of the two vaccines against wild strains of the new coronavirus, published in December last year, showed efficiencies of 95% and 90%, respectively.
However, studies have shown that the protective efficacy of one dose of the vaccine is only 30.7% (the efficacy of the two vaccines is similar). This suggests that the long-interval vaccination strategy recommended by countries earlier this year when there was a shortage of vaccines is not desirable.
The editorial issued by NEJM at the same time pointed out that we cannot randomize the variant strains. Therefore, even if there are limitations, real-world studies are still extremely important for judging the protective efficacy of vaccines. This paper is the first research paper on the protective efficacy of vaccines against delta variants that has been officially published after peer review.
The Delta variant of the new coronavirus was detected in India in December 2020 and has now spread to more than 100 countries. The spike protein of the Delta mutant virus has mutations of T19R, Δ157-158, L452R, T478K, D614G, P681R and D950N. These mutations may affect the body’s immune response, and the P681R mutation can promote virus replication, thereby increasing the viral load and spreading ability.
In this real-world study, the author used two research methods. The first is a case-control analysis of negative detection. In short, people who seek medical treatment (or screening) and are tested positive for the new coronavirus are treated as patients. At the same time, people who have the same conditions but tested negative are selected as the control group. The vaccination status of these two groups of people is clear, and then The ratio of the vaccination status of the two groups was used to judge the protective efficacy of the vaccine against the Delta mutant strain. This method can eliminate the biases caused by the enthusiasm for seeking medical attention, the accessibility of testing, and the confirmation of cases.
The second method is to compare the relative proportions of patients infected with the Delta variant strain and the major epidemic strain (Alpha) in the UK during the study period among patients with different vaccination status. This method is based on the assumption that if the vaccine is effective and has the same protective efficacy against each strain, then the proportion of infection cases for each variant strain should be similar in the unvaccinated and vaccinated population. Conversely, if the vaccine’s protective efficacy against the Delta variant strain is weaker than that against the Alpha variant strain, compared with the proportion of people infected with the Delta variant strain in the unvaccinated population, those infected with the Delta variant strain 3 weeks after vaccination The proportion should be higher than the former proportion.
The development of this research relies on the UK’s comprehensive national vaccination registration system, widely accessible virus testing and large-scale viral genome sequencing. The patients included in the analysis met the following conditions: age ≥16 years old, symptomatic COVID-19 patients confirmed to have Alpha or Delta infection, and had been vaccinated with ChAdOx1 nCoV-19 adenovirus vector vaccine or BNT162b2 mRNA vaccine. The study finally included 19,109 patients who had undergone viral gene sequencing, of which 14,837 samples were alpha variants and 4272 samples were Delta variants.
Demographic analysis shows that most patients with Delta variants have a history of recent travel abroad, and most of them have recent onsets (18-20 weeks in 2021). The proportion of patients located in northwestern England, London or eastern England is relatively high, and they are of Indian origin and Pakistani origin. The proportion of Asian or other Asians is higher.
A comprehensive analysis of all vaccinated populations showed that after the first dose of vaccine, the protective efficacy against the Delta variant strain was 30.7%, and the protective efficacy against the Alpha variant strain was 48.7%. The protective efficacy of the two vaccines after the first dose was similar. The absolute difference in the protective efficacy of the BNT162b2 vaccine against Alpha and Delta variants was 11.9%, while the absolute difference between ChAdOx1 nCoV-19 was 18.7%.
After the second dose of vaccine, the absolute difference in vaccine protective efficacy was reduced to 6 to 8 percentage points. A comprehensive analysis of all vaccinated populations showed that the protective efficacy against Alpha variants was 87.5%, and the protective efficacy against delta variants was 79.6%.
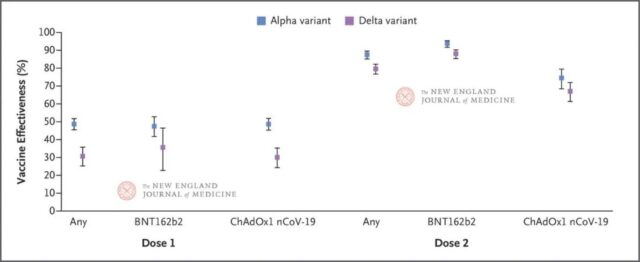
The protective efficacy of different vaccines and different doses against Alpha and Delta variants (DOI: 10.1056/NEJMoa2108891)
In addition, the results of this article once again confirmed that the protective efficacy of the BNT162b2 vaccine is better than that of the ChAdOx1 nCoV-19 vaccine.
However, the author pointed out in the supplementary discussion that the second dose of ChAdOx1 nCoV-19 was administered later than BNT162b2. Therefore, the difference between the two vaccines may be due to the fact that it takes more than 2 weeks for the former to exert its maximum protective effect. Moreover, due to the small number of patients and short follow-up time, the data collected in the current study cannot calculate the protective efficacy of the two vaccines against severe diseases.
Small-scale trials have shown that the serum of patients after vaccination with these two vaccines can neutralize the Delta mutant strain; and the mRNA vaccine can continue to provide protection against the mutant strain. A similar Canadian study published on this website recently showed that BNT162b2, mRNA-1273, and ChAdOx1 nCoV-19 vaccines can prevent symptomatic COVID-19 caused by Alpha, Beta (B.1.351), Gamma, and Delta variants.
The NEJM editorial pointed out that although the test-negative case-control study design adopted in this study can reduce some biases, other bias factors cannot be ignored.
For example, in this study, the vaccination methods of the two vaccines at different time points are different, the vaccine supply of different medical institutions is different, and the vaccination of different age groups is not the same, so accurate comparison is more difficult. These unconsidered biases are likely to make the results represented by the confidence interval more uncertain.
From the perspective of clinical treatment, the protective effect of vaccines is reflected in the prevention of severe diseases and deaths. However, from a public health perspective, reducing the infection rate is equally important. As of June 19, 2021, in the UK alone, the new coronavirus vaccine has prevented 7,200,000 cases and 27,000 deaths. Therefore, the current strategy is to get as many people as possible vaccinated as soon as possible.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



