The world’s first oral anti-coronavirus drug is about to be approved
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
The world’s first oral anti-coronavirus drug is about to be approved
The world’s first oral anti-coronavirus drug is about to be approved. On August 9, 2021, the Australian Medicines Agency granted Merck & Co. Molnupiravir an interim decision so that Merck can register and apply for the listing of new drugs of Molnupiravir in Australia.
In the early stage of infection, the virus replicates rapidly, and the host’s immune system has not yet had enough time to establish an immune defense, so the best time for antiviral drug therapy to block the virus is a few days before the infection. Molnupiravir is designed for outpatient use in the early stage of the disease and is an oral capsule, so the number of beneficiaries will be greatly increased.
Molnupiravir is a broad-spectrum antiviral drug against RNA viruses. I have made a lot of introductions since the beginning of last year. I think this small antiviral molecule has good antiviral activity against SARS-CoV-2.
Uploaded on medRxiv on June 21, 2021, in a clinical trial led by the University of North Carolina (UNC), 3 days after treatment, the probability of separable replicative virus in the 800 mg group of Molnupiravir treatment was significantly lower than that of the placebo group (1.9% vs. 16.7%, p = 0.02), this is a gold standard for testing the infectivity of viruses. After 5 days of treatment, replication viruses could not be isolated in patients in the 400 and 800 mg groups, and replication viruses could be isolated in 11.1% of patients in the placebo group (p = 0.03).
Currently the most promising small molecule anti-coronavirus drug, phase 2 clinical trial results are exciting
On August 11, 2021, Max Planck Institute published a good article in Nature SME, reporting the molecular mechanism of action of the potential high-efficiency antiviral drug Molnupiravir.
This study describes the mechanism by which Molnupiravir induces mutations in viral RNA replication. The active form of Molnupiravir is β-D-N4-hydroxycytidine (NHC)-triphosphate. The RdRp of the virus will mistakenly use NHC-triphosphate instead of cytidine triphosphate or uridine triphosphate as a catalytic substrate.
NHC will cause viral RNA replication to incorporate a large amount of A and G, resulting in mutations in the RNA product. Structural analysis of the RdRp-RNA complex containing the mutagenized product showed that NHC can form a stable base pair with G or A in the active center of RdRp, which explains how the drug evades proofreading and synthesizes mutant RNA.
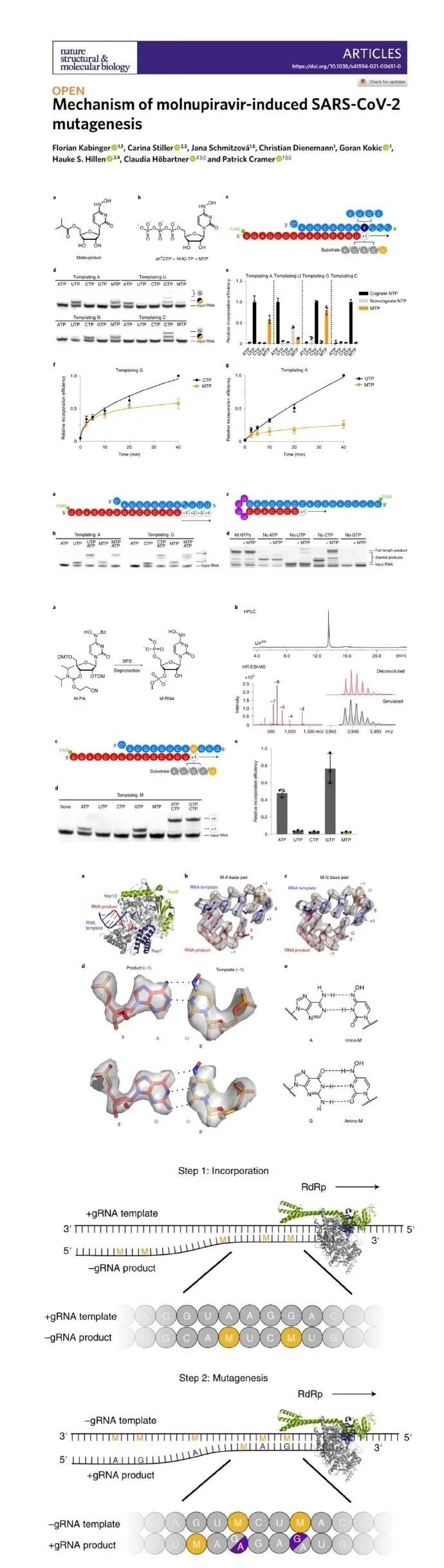
This two-step mutagenesis mechanism may be applicable to various viral polymerases, so Molnupiravir may have broad-spectrum anti-RNA virus activity.
In June 2021, the US Biden government promised that if the US FDA grants emergency use authorization for Molnupiravir, it will purchase 1.7 million courses of Molnupiravir, valued at approximately US$1.2 billion.
As early as February 4, 2020, when we were discussing Remdesivir’s treatment of COVID-19 pneumonia, we mentioned:
The combination of antiviral therapy and monoclonal antibodies is an ideal method for the treatment of COVID-19 pneumonia.
Is Remdesivir, which is undergoing clinical trials of COVID-19 pneumonia, a magic drug?
At the same time, the challenge faced by the new coronavirus and other viruses that cause severe pneumonia is the treatment time window. That is, the earlier the treatment, the better the effect.
This also means that oral medications are required.
In terms of monoclonal antibody development for the treatment of mild to moderate COVID-19, three drugs have been approved:
A, Regeneron’s monoclonal antibody cocktail REGEN-COV;
B, the monoclonal antibody Sotrovimab (VIR-7831) jointly developed by Vir Biotechnology and GlaxoSmithKline in the United States;
C. Eli Lilly’s monoclonal antibody Bamlanivimab was the first to be approved, but the emergency use authorization was cancelled due to the mutant strain; later bamlanivimab and etesevimab were approved again, but the distribution was suspended again on June 25.
In addition, currently available monoclonal antibodies are all injections, which severely restricts clinical use.
The remdesivir developed by Gilead is also an injection, so it cannot be used early in the outpatient clinic; with July 30, 2021, Gilead announced that it will stop clinical trials of remdesivir inhalation and is currently oral in the early treatment of COVID-19 The hope of antiviral agents fell on Molnupiravir.
With many expectations, I wish Molnupiravir to be listed soon.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



