How many people have allergic reactions to Pfizer or Moderna vaccines?
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
How many people have allergic reactions to Pfizer or Moderna COVID-19 vaccines?
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
How many people have allergic reactions to Pfizer or Moderna COVID-19 vaccines?
The large-scale study of 39,000 doses of vaccinations based on the mRNA COVID-19 vaccine tells you the answer: are there many allergic reactions to the COVID-19 vaccine? Can it be treated?
The United States has reported the first severe allergic reaction to the Pfizer COVID-19 vaccine, and claimed that people who had a severe allergic reaction to the first shot of the COVID-19 vaccine should not receive a second shot.
Recently, a study published in the journal “JAMA Network Open” showed that 22 potential allergic reactions to 38,895 doses of mRNA-based COVID-19 vaccine were analyzed, and it was found that they may be sensitive to PEG before vaccination, and the mRNA itself did not cause allergic reaction.
Individuals who respond to the first dose of the vaccine can safely receive the second dose under medical supervision.
According to reports, the United States has experienced the first severe allergic reaction to the Pfizer COVID-19 vaccine. Two medical staff in the same hospital in Alaska received first aid after being vaccinated with Pfizer’s COVID-19 vaccine.
A medical worker who injected the Pfizer vaccine at the Barrett Regional Hospital developed severe allergies 10 minutes after the vaccination, a rash on his face and body, shortness of breath and a rapid heartbeat. The director of the emergency department of the hospital stated that the person received standard allergy treatment, but his symptoms repeated and he was sent to the intensive care unit for observation.
The second medical staff who received first aid received the injection on the 16th. Ten minutes after the vaccination, symptoms such as ocular swelling, dizziness and itchy throat appeared. He was then sent to the emergency room for treatment.
However, the hospital stated that his symptoms were not allergies, and he was allowed to leave the hospital within an hour. As of the evening of the 16th, this hospital has received 144 doses of Pfizer vaccine. Anne Zink, chief medical officer of Alaska, said there are no plans to change the vaccination plan at this time.
Although the Pfizer vaccine has proven to be safe in clinical trials involving 44,000 people, with an effective rate of about 95%, the Alaska case may exacerbate concerns about possible side effects.
Experts say that these issues may prompt people to demand stricter guidelines to ensure careful monitoring of adverse reactions. (Source: New York Times)
The CDC in the United States once stated in a COVID-19 vaccination guidance that people who have a severe allergic reaction to the first shot of the COVID-19 vaccine should not receive a second shot.
The CDC also stated that after the first batch of COVID-19 vaccines were sent to all states in the United States on the 13th, 272,000 people have been vaccinated across the United States, and 6 people developed severe allergic reactions during the observation period and received timely treatment.
Regarding allergies, Chinese experts pointed out that regarding the contraindications of vaccination allergy, China’s “COVID-19 Vaccination Technical Guidelines” stipulates two points:
- One is allergic to vaccine ingredients;
- The second is that serious allergic reactions have occurred in the past when the COVID-19 vaccine was used.
There are two kinds of allergies that cannot be vaccinated. People who have food allergies, drug allergies, cosmetics allergies, and mite allergies are usually vaccinated. “
In addition, the suspected allergy is related to vaccination, and it is also necessary to determine whether it is a general reaction or an abnormal reaction. Every region has a vaccination abnormal response investigation and diagnosis expert group and a vaccination abnormal response identification group, which will make a comprehensive judgment based on the characteristics of the vaccine, the clinical manifestations and past history of the recipient, the diagnosis and treatment process, and the vaccination situation, etc., to determine whether it is Allergic reactions caused by vaccination. “
Regarding the issue of allergies to the COVID-19 vaccine, a recent study by the Stanford University School of Medicine showed that allergic reactions to the new mRNA-based COVID-19 vaccine are rare, usually mild and treatable, and they should not prevent people from being vaccinated. vaccine. The results of the study were published in the journal “JAMA Network Open” on September 17.
Kari Nadeau, MD, senior author of the study and professor of pediatric food allergy, immunology and asthma at the NADDISY Foundation, said: “We want to know the allergens of the new vaccines and understand the causes of them.”
This study analyzed the 22 potential allergic reactions of the first batch of 39,000 doses of Pfizer and Moderna’s COVID-19 vaccine received by the Stanford University health care institution shortly after receiving the emergency use authorization from the U.S. Food and Drug Administration (FDA).
Most people who responded in the study were allergic to components that help stabilize the new coronavirus vaccine; they did not show allergies to vaccine components that are immune to the SARS-CoV-2 virus. In addition, these allergic reactions occur through indirect activation of allergic pathways, which makes them easier to alleviate than many allergic reactions.
Nadeau, director of the Sean Parker Center for Allergy and Asthma Research at Stanford University, said: “It is great to know that these reactions are manageable. Allergic reactions to these new vaccines are rare, and if they do occur, there are ways to control them. “
The lead author of the study is a former postdoctoral scholar, Dr. Christopher Warren, who is now an assistant professor at Northwestern University’s Feinberg School of Medicine.
Nadeau also said that the study also suggests how vaccine manufacturers can reformulate vaccines to reduce the possibility of allergic reactions.
Give instructions on protein manufacturing
The mRNA-based COVID-19 vaccine provides immunity through small fragments of mRNA, which encodes the molecular instructions for making proteins. Because the mRNA in the vaccine is fragile, it is encapsulated in bubbles of lipids (fatty substances) and sugar to maintain stability.
When the vaccine is injected into a person’s arm, the mRNA can enter nearby muscles and immune cells, and then produce a non-infectious protein similar to the surface of the SARS-CoV-2 virus. These proteins trigger an immune response, enabling the human immune system to recognize and defend against viruses.
According to the Federal Vaccine Adverse Event Reporting System, the estimated incidences of severe vaccine-related allergic reactions (severe allergic reactions requiring hospitalization) for Pfizer and Moderna vaccines are 4.7 and 2.5 per million doses, respectively.
However, the federal system did not capture all allergic reactions to vaccines, and often ignored those mild or moderate allergic reactions.
In order to get a more comprehensive understanding of how common and severe the allergic reaction to the new vaccine is, the research team checked the medical records of medical workers: between December 18, 2020 and January 26, 2021, they were in Stanford Medicine. The hospital received 38895 doses of mRNA-based COVID-19 vaccine.
These vaccinations include 31635 doses of Pfizer vaccine and 7,260 doses of Moderna vaccine.
Researchers searched the medical records of allergic reactions of vaccinators and determined which reactions were related to the vaccine.
Twenty-two subjects, 20 of whom were women, may have allergic reactions, which means that specific symptoms began to appear within 3 hours of receiving the injection.
Researchers looked for the following symptoms in the recipient’s medical records: hives; swelling of the mouth, lips, tongue, or throat; shortness of breath, wheezing, or chest tightness; or changes in blood pressure or loss of consciousness.
Only 17 of 22 subjects met the diagnostic criteria for anaphylaxis.
Three subjects received adrenaline therapy, usually for stronger allergic reactions. All 22 people recovered.
Among the 22 subjects, 15 had a history of allergic reactions recorded by a doctor, including 10 allergic to antibiotics, 9 allergic to food, and 8 allergic to non-antibiotic drugs. (Some recipients have more than one type of allergy.)
The researchers conducted follow-up laboratory tests on 11 people to determine what type of allergic reactions they had and what triggered their allergies: was it the inert sugar or lipid component in the foam, or other components in the vaccine?
Study participants underwent a skin prick test in which clinicians injected small amounts of potential allergens-lipids, sugars (polyethylene glycol or polysorbate) or whole vaccines into the skin.
The skin prick test can detect allergic reactions mediated by an antibody called immunoglobulin E (IgE); these reactions are usually associated with the most severe allergies.
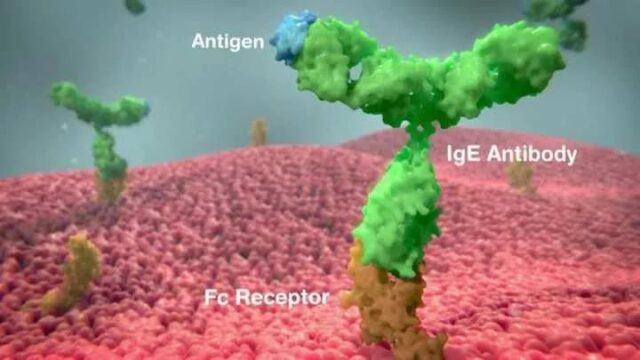
In the skin prick test, no subject reacted to the inert ingredients in the vaccine, and only one subject’s skin reacted to the entire COVID-19 vaccine.
Subsequent blood tests showed that the vaccinators did not have significant levels of IgE antibodies against the vaccine components.
Since the skin test cannot explain the mechanism of the receptor’s allergic reaction, the researchers proceeded to another diagnostic test. Vaccine recipients provided blood samples to test for allergic activation of immune cells called basophils.
The blood samples of 10 of the 11 participants showed a reaction to polyethylene glycol (PEG), an inert ingredient used in the Pfizer and Moderna vaccines.
In addition, when the entire mRNA vaccine was mixed with their own basophils, the basophils of all 11 subjects were activated.
The blood of all 11 subjects contained high levels of anti-PEG IgG antibodies; in some cases, IgG antibodies helped to activate basophils. This finding suggests that these individuals may be susceptible to PEG prior to vaccination. sensitive.
Nadeau said: “It seems that the mRNA itself does not cause allergic reactions.”
In addition, the data shows that the reaction to the COVID-19 vaccine is usually not the most serious allergic reaction, which is good news in terms of vaccine safety.
Allergic reactions mediated by IgG and basophils can be controlled with antihistamines, fluids, corticosteroids and close observation, which means that many individuals who respond to the first dose of the vaccine can be safely under medical supervision To receive the second dose of vaccine.
PEG is widely used as a stabilizer for household products, cosmetics and medicines, and women are more likely to be exposed to large amounts of this substance, which may explain why more vaccine allergies appear among women. (Repeated exposure to a substance can sometimes sensitize the immune system and cause allergies.)
Nadeau said: “Since most of the reactions are to polyethylene glycol PEG rather than the active ingredient of the vaccine, vaccine manufacturers are likely to reformulate the vaccine with different stabilizers, which are unlikely to cause allergies.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



