Neoantigens in Tumor Immunotherapy and related cancer vaccines
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Neoantigens in Tumor Immunotherapy and related cancer vaccines
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Neoantigens in Tumor Immunotherapy and related cancer vaccines
Preface
In recent decades, tumor immunotherapy has shown great anti-cancer potential, and the immune system is activated to target malignant tumors.
The clinical outcome of tumor immunotherapy depends on the presence of tumor-specific antigens, which is the core of tumor immunotherapy based on T lymphocytes.
Generally, TCR on the surface of CD4+ and CD8+ T lymphocytes recognize antigens ( Ag ) or epitopes presented by major histocompatibility complex ( MHC ) class I and II molecules, respectively.
When the MHC-Ag-TCR tertiary complex is formed as a recognition signal and a costimulatory signal ( the interaction between CD28-CD80/CD86 ), they can trigger signal transduction in T cells and then destroy target cells.
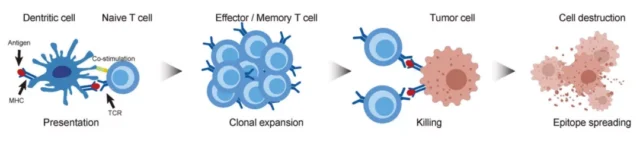
There are three main sources of tumor antigens: tumor-associated antigens ( TAAs ), oncogenic virus-derived antigens, and tumor-specific antigens ( TSA, neoantigens ).
The following table summarizes the characteristics of the three tumor antigens.I
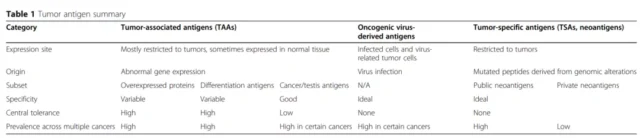
Neoantigens are tumor- specific antigens derived from non-synonymous mutations and are very attractive targets for tumor immunotherapy.
Due to the development of next-generation sequencing technology and the application of machine learning algorithms, it has become possible to calculate and predict neoantigens by describing genetic changes in tumor tissues, abnormal post-transcriptional mRNA processing, and abnormal mRNA translation events. Therefore, neoantigen-based therapies, such as cancer vaccines, have been extensively tested in clinical trials and have proven to have good safety and effectiveness, opening up a new era of tumor immunotherapy.
Identification of neoantigens
Unlike TAA, TSA/neoantigen is a mutant peptide obtained from the genetic changes of the tumor genome. It is specifically expressed in tumor cells and not present in normal tissues. Therefore, it shows high tumor specificity and reduces non-targeting. toxicity. Personalized immunotherapy based on neoantigens can stimulate a powerful anti-tumor immune response against tumor cells.
New antigen identification relies on high-throughput sequencing data from DNA and RNA samples from tumors and normal tissues. By using bioinformatics methods to analyze whole exome sequencing ( WES ) and mRNA transcriptome sequencing ( RNA Seq ) data, it is possible to determine the DNA and RNA level mutations that may lead to neoantigenic epitopes.
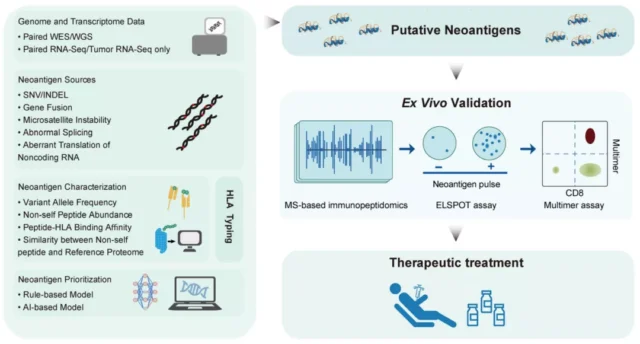
Generally, non-synonymous single nucleotide variation ( SNV ) and DNA insertion or deletion are the main sources of neoantigen prediction. However, focusing only on these two types of mutations tends to underestimate the potential neoantigens of the tumor. Neoantigens can also be derived from many other types, including
(1) gene fusion events;
(2) splicing site mutations ( SCM );
( 3) mRNA intron retention;
(4) endogenous retrotransposition.
It is worth noting that neoantigens derived from other types of mutations ( such as gene fusion and SCM ) tend to be more immunogenic than neoantigens derived from SNV.
In addition, important data related to the immunogenicity of neoantigens also include: (1) the affinity between the patient’s mutant peptide and HLA allele; (2) the binding stability of the peptide HLA complex and (3) the host gene Express. In addition to the features from WES and RNA-Seq data, other features describing the recognition potential between mutant peptides and T cell receptors can also be incorporated into the neoantigen prediction system.
Synthetic long peptide (SLP) vaccine
Neoantigen-based tumor treatments mainly include synthetic long peptide ( SLP ) vaccines, nucleic acid ( DNA/mRNA ) vaccines, dendritic cell ( DC )-based vaccines, neoantigen-specific TCR-T cell-based treatments, and public new Antigen-related bispecific antibodies. Clinical trials based on these personalized neoantigen therapies have been conducted in advanced cancer patients worldwide.
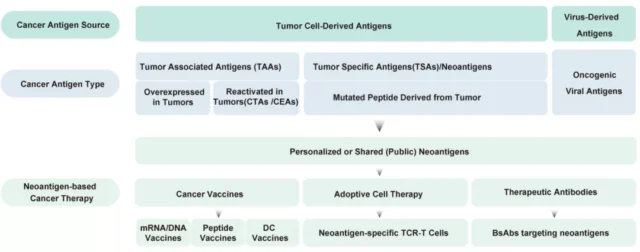
At present, synthetic peptide-based neoantigen vaccines have been used in multiple clinical trials, and some clinical trials have shown the anti-tumor efficacy of peptide tumor neoantigen vaccines. NeoVax is an individualized neoantigen peptide vaccine for patients with melanoma.
In a phase 1 clinical trial ( NCT01970358 ), patients with high-risk melanoma at stage IIIB/C and IVM1a/b were selected pathologically, and the median postoperatively A few 18 weeks later, he received NeoVax treatment.
Individualized peptide vaccines targeting 20 new antigens are formulated from poly-ICLC, which is a synthetic double-stranded RNA ( dsRNA ) mimic that can stimulate TLR3 and MDA5.
By stimulating MDA5, Poly-ICLC can effectively induce IFN-I and IL-15. It can also promote T cell expansion and enhance T cell infiltration, making it an effective adjuvant for peptide cancer vaccines.
In 2021, the study announced the follow-up results of 8 melanoma patients participating in this trial, with an average follow-up time of 55 months. The results showed that all 8 patients were alive and 6 patients showed no signs of active disease.
Tests on T cells in the peripheral blood of patients showed that after NeoVax vaccination, the neoantigen-specific T cell response of melanoma patients continued for several years, and the neoantigen-specific T cells showed a memory phenotype.
In addition, the study also found that NeoVax can induce the spread of epitopes that respond to T cells, which also means that the range of tumor-specific cytotoxicity is broadened.
However, three patients in this study relapsed 26, 40, and 40 months after vaccination, and the other two patients relapsed shortly after vaccination, but after treatment with the anti-PD-1 inhibitor pembrolizumab, they quickly achieved complete remission.
A phase Ib clinical trial ( NCT02897765 ) studied the efficacy of a personalized neoantigen vaccine NEO-PV-01 combined with PD-1 blockers in the treatment of patients with advanced melanoma, non-small cell lung cancer or bladder cancer. In this experiment, the neoantigen peptide and the adjuvant poly ICLC were mixed subcutaneously, and Nivolumab was used continuously during the vaccination period.
The results showed that the overall response rates of patients with melanoma, non-small cell lung cancer, and bladder cancer were 59%, 39%, and 27%, respectively, and the median progression-free survival ( PFS ) was 23.5 months, 8.5 months, and 5.8 months, respectively ; The 1-year overall survival rate is 96%, 83% and 67%, and these data are not lower than the historical data of PD-1 antibody monotherapy.
Epitope spread has also been observed after vaccination and is associated with longer PFS.
Nucleic acid (DNA/mRNA) vaccine
DNA/mRNA-based neoantigen vaccines work through similar steps as peptide-based vaccines, except for additional translation and/or transcription steps in DC.
Compared with peptides, nucleic acid preparations contribute to the sustained and effective expression of antigens and immune stimulation. Importantly, nucleic acid preparations produce antigen peptides from cells, avoiding expensive and complicated protein purification, and equipping proteins with natural post-translational modifications.
Nucleic acid vaccines have advantages in efficacy, shortening design and manufacturing time, and production scalability and reliability. Currently, new antigen-targeted DNA/mRNA cancer vaccines have also been tested in various clinical trials.
In a phase 1b clinical trial ( NCT03289962 ), a personalized RNA liposomal neoantigen-based vaccine RO7198457 was tested in patients with advanced solid tumors who had previously received multiple treatments.
The vaccine encodes as many as 20 new types. antigen. A total of 29 patients received RO7198457 monotherapy, and 132 patients received RO7198457 combined with the anti-PD-L1 antibody atezolizumab.
According to the data of patients who have received at least one tumor evaluation, the trial showed that the ORR of monotherapy was 4% (1/26) and 40% of SD ( 9/26 ), the ORR of combination therapy was 8% ( 9/108 ), and SD It is 49% ( 53/108 ). In most patients in both groups, RO7198457 induced a neoantigen-specific T cell response.
In another phase 1 clinical trial ( NCT03313778 ), another RNA-based neoantigen vaccine, mRNA-4157, was tested.
Of the 79 patients who received mRNA-4157 treatment, 16 received monotherapy and 63 were combined with pembrolizumab.
mRNA-4157 is safe and well tolerated. 3 cases of complete remission ( CR ) and 8 cases of partial remission (PR) were observed in the combined administration group .
Especially in 10 HNSCC patients, the ORR was 50% and mPFS was 9.8 months, while the ORR of pembrolizumab monotherapy was only 14.6% and mPFS was 2 months.
DC vaccine and neoantigen specific TCR-T
Recently, a phase I clinical trial ( NCT02956551 ) studied the safety and effectiveness of a personalized neoantigen autologous DC vaccine ( Neo DCVac ) in the treatment of 12 cases of severely metastatic lung cancer. The results showed that the overall safety of the vaccine was good, with ORR of 25%, DCR of 75%, mPFS of 5.5 months, and median overall survival ( mOS ) of 7.9 months. When used in combination with ICI therapy, Neo-DCVac shows a synergistic therapeutic effect. These results indicate that Neo DCVac can induce specific T-cell immunity, and this trial proved the efficacy of neoantigen-based DC vaccines for cancer patients for the first time.
TCR-based engineered T cell therapy is another option for neoantigen applications. Compared with the DC vaccine trial, there are currently no clinical trial results of neoantigen-specific TCR-T cell therapy, and only case reports have been published.
These cases included a patient with metastatic colorectal cancer who received KRAS-G12D targeted TIL therapy and a patient with chemotherapy-refractory HR-positive metastatic breast cancer who received TIL therapy.
TIL targeted SLC3A2, KIAA0368, CADPS2, and CTSB, respectively. Four mutations in the distribution. Both treatments achieved clinical efficacy.
Breast cancer patients who received both IL-2 and ICI combined therapy showed complete and lasting regression for more than 22 months, but only received KRAS-G12D targeted TIL therapy for metastasis Patients with colorectal cancer progressed 9 months after treatment.
One of the biggest challenges of neoantigen-based T cell therapy is to identify and isolate these mutation-specific TILs and TCRs.
In addition, neoantigen-based DC and TCR-T therapies should pay attention to potentially fatal side effects including cytokine release syndrome ( CRS ).
Cancer vaccines based on public neoantigens
Compared with purely personalized neoantigens, public or shared neoantigens are derived from driver mutations in oncogenes or other hotspot mutations in the genome. They are characterized by immunogenic epitopes present in a subset of patients with specific cancer subtypes.
Therefore, the discovery of public neoantigens relies on the analysis of individualized neoantigens from a considerable patient pool.
A major advantage of public neoantigens is that they can be quickly applied to cancer patients, especially advanced cancer patients and patients with narrow treatment windows. In addition, cancer vaccines with public neoantigens will reduce the cost of treatment.
An example of a public neoantigen is the G12D mutation on KRAS, which is common in pancreatic cancer, colon adenocarcinoma, non-small cell lung, and colorectal cancer. Similarly, TP53 is a well-known tumor suppressor gene, which is widely mutated in a large number of cancers, has a wide range of hotspot mutations, and is shared by many cancers.
Currently, most clinical trials for public neoantigens are still in progress, and some early trials have reported good results. In an open-label, single-arm phase I clinical trial ( NCT01250470 ), the safety and effectiveness of survivin peptide ( BIRC5 ) in 9 patients with relapsed malignant glioma were tested .
The survivin peptide is 15 amino acids long and contains different The immunogenic epitope of 8 to 10 amino acids has the same C57M mutation. The results show that some patients have achieved partial remission or have been stable for at least 6 months.
In another experiment with a similar design ( NCT02261714 ), the KRAS mutant peptide TG01 ( containing seven known oncogenic mutations ) was used as a vaccine in combination with granulocyte-macrophage colony stimulating factor ( GM-CSF ) , For 32 patients with stage I or II pancreatic cancer who underwent surgical resection ( R0 or R1 ), of which 93.75% carried detectable KRAS mutations. The results show that the safety of the low-dose group is better. Both doses produced a strong cellular immune response, and the 2-year and 3-year survival rates of subjects were approximately 72 and 37%, respectively. In short, the public neoantigen vaccine has proven its great value as a cancer treatment target.
Bispecific antibodies related to public neoantigens
Bispecific antibody is a type of antibody that can be directed against two antigens at the same time.
It connects effector cells and target cells so that the effector cells can give full play to the tumor-killing effect. Recently, a preclinical study of a bispecific antibody against the TP53 gene hotspot mutation R175H neoantigen was reported.
The single-chain antibody targeting p53R175H/HLA-a*02:01 was linked to the anti-CD3e single-chain antibody ( UCHT1 ). This T cell-directed neoantigen bispecific antibody shows strong anti-tumor immune activity in vitro and in vivo.
Summary
In recent years, anti-tumor therapies based on personalized or public neoantigens have made great progress in the identification, prediction or screening of neoantigens, and treatment options.
Neoantigen vaccines that use mRNA as a carrier and neoantigen vaccines that target public neoantigens have shown better clinical characteristics and druggability, and have aroused great interest in the development of next-generation precision tumor immunotherapy.
However, there are still some challenges in neoantigen-based therapy, and several aspects of neoantigen vaccines need to be optimized to achieve better clinical response.
First of all, the time required to identify and manufacture neoantigens is relatively long, at least 6-8 weeks. In view of the low accuracy of currently available neoantigen prediction algorithms, a lot of effort is required to use machine learning platforms to improve the accuracy of neoantigen prediction .
Second, it is necessary to test and evaluate the best neoantigen formulation and corresponding modifications, the neoantigen delivery system used, the route, and the safe and effective dosage in clinical trials.
In addition, the combination of neoantigen vaccines with TAAs and other immunomodulator therapies has shown good synergistic therapeutic effects.
Therefore, the use of neoantigen vaccines and radiotherapy, chemotherapy or ICIs and TME immunomodulators should be further evaluated in future studies.
It is believed that the neoantigen vaccine, as a brand-new and extremely promising technology, will solve these problems, and it will surely occupy a place in the field of tumor immunotherapy in the future.
references:
1.Targeting neoantigens forcancer immunotherapy. Biomark Res. 2021; 9: 61.
Neoantigens in Tumor Immunotherapy and related cancer vaccines
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



