Polypeptide COVID-19 vaccine is more potential than mRNA vaccine?
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Polypeptide COVID-19 vaccine is more potential than mRNA vaccine?
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Polypeptide COVID-19 vaccine is more potential than mRNA vaccine?
Nature: Polypeptide COVID-19 vaccine is here. Will it have more potential than mRNA vaccine in the future?
On November 23, 2021, Nature published a phase I clinical trial of a protein-based COVID-19 vaccine . The results show that it has good effectiveness and safety, and the detailed data has not been disclosed yet.
This vaccine is different from other new coronavirus vaccines in the past in that it expresses pathogen antigen proteins through genetic engineering to prepare vaccines. The main advantages of this type of vaccine are simple manufacture and low cost.

At present, in the research and development route of the COVID-19 vaccine, in addition to the traditional inactivation and adenovirus technology, there are also recent popular mRNA and DNA technologies, and the latest protein technology may also be a popular technology for the future development of vaccines including the COVID-19 vaccine. .
The clinical trial disclosed this time is called CoVac-1 , which is a peptide-based vaccine candidate that can induce an immune response to the new coronavirus . The candidate vaccine induces T cell immunity-an important response to control the virus, or may help people with immunodeficiency.
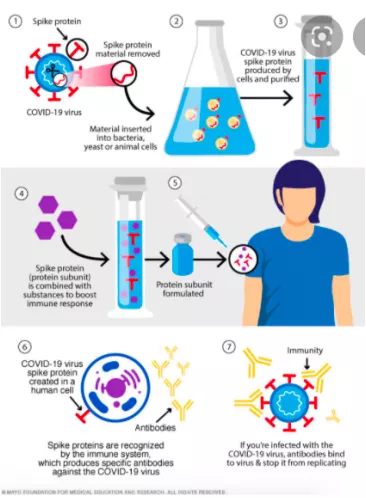
The design principle of protein vaccine, the picture comes from mayo
In the human body, T cells play an important role in fighting viruses and other pathogens by attacking infected cells or promoting B cells to produce protective antibodies. T cell immunity is particularly important for patients with B cell defects (such as cancer patients) .
The following is the data disclosed this time about the effectiveness and safety of the protein vaccine.
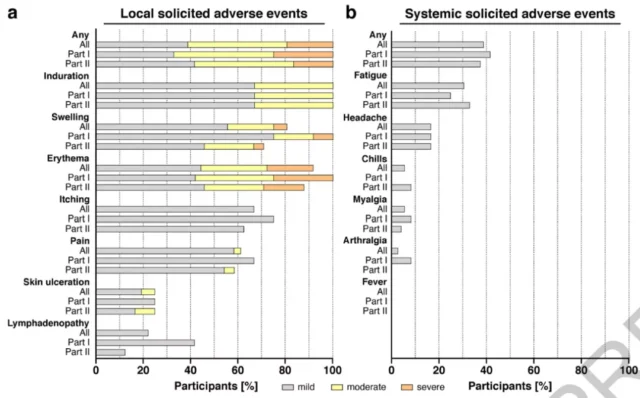
Statistics of side effects caused by protein vaccines
According to the relevant data of the paper, the goal of a single injection of CoVac-1 is to induce long-term, similar to natural infection of the new coronavirus (SARS-CoV-2) T cell immunity.
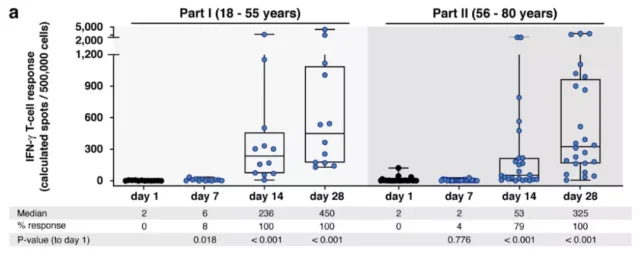
Immune response at different ages after vaccination
In the first clinical evaluation of CoVac-1, Juliane Walz and colleagues recruited 36 participants, aged 18-80, to receive a dose of peptide vaccine. 28 days after vaccination, SARS-CoV-2 specific T cell responses were observed in all participants, and the effect lasted for at least 3 months.
Researchers have found that the T cell response induced by the vaccine is better than the response induced by natural infection with the new coronavirus, and the major new coronavirus variants (Alpha, Beta, Gamma and Delta) currently under attention do not change the T cell response induced by the vaccine. That is, even if the new coronavirus is mutated, the vaccine is still effective against this mutant strain.
Finally, the authors of the paper concluded that the vaccine has good safety among all trial participants and induced T cell responses. In the future, this vaccine will continue to be evaluated through subsequent trials.
At present, at least 6 companies around the world are developing protein COVID-19 vaccines.
Developed by Novavax, an American biological company founded in 1987, before 2020, the company mainly developed vaccines in the fields of Ebola, influenza, and respiratory syncytial virus. After the outbreak of the COVID-19 in 2020, the company re-positioned and began to develop a COVID-19 vaccine. Unlike the traditional vaccine technology and the vaccine prepared by the recently popular mRNA technology, the company uses peptides to prepare the COVID-19 vaccine.
The clinical development of the recombinant protein COVID-19 vaccine in China is also advancing. In March of this year, the results of the Phase I Phase II clinical trial of the recombinant protein new coronavirus vaccine jointly developed by the Institute of Microbiology of the Chinese Academy of Sciences and Anhui Zhifei Biotechnology were released.
According to related reports, the results of the recombinant protein showed that 97% of the participants who received 3 doses of 25 micrograms vaccine produced neutralizing antibodies that can block the live virus, and the level of neutralizing antibodies exceeded the serum of recovered patients. March 10 this year Relevant departments evaluate and demonstrate that they agree to emergency use. Currently, the recombinant protein vaccine is undergoing international multi-center phase III clinical trials in Uzbekistan, Indonesia, Pakistan and Ecuador.
Of course, the protein COVID-19 vaccine has no shortcomings. According to reports, protein-based vaccine design is slower than other vaccine technologies. The main reason is that large-scale production of purified protein involves many steps, and each step must be optimized, which leads to slow progress.
But fortunately, we have a new preventive method against the new coronavirus, and protein vaccine technology will also have more applications in other infectious diseases in the future.
Paper link:
https://www.nature.com/articles/s41586-021-04232-5
Polypeptide COVID-19 vaccine is more potential than mRNA vaccine?
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



