Why many questions about COVID-19 oral drugs still need to be answered?
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Why many questions about COVID-19 oral drugs still need to be answered?
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Why many questions about COVID-19 oral drugs still need to be answered?
Recently, Pfizer announced that it has sought emergency use authorization for the oral antiviral therapy Paxlovid from the US FDA for the treatment of patients with mild to moderate COVID-19.
At the same time, it has reached a licensing agreement with the United Nations-supported Medicines Patent Pool organization to expand the use of this therapy in low- and middle-income countries.
Previously, Molnupiravir, jointly developed by Merck & Co. (MSD) and Ridgeback Biotherapeutics, has been approved by the UK regulatory agency.
These two oral antiviral therapies have received widespread attention recently. This article will combine public information to introduce to readers the relevant information of these two oral antiviral therapies in detail.
Oral antiviral drugs are the latest tool in the “toolbox” of fighting the epidemic. Industry experts said that their emergence plays an important role in treating patients with COVID-19 disease and preventing the occurrence of severe illness and death of patients.
Oral antiviral drugs can be taken directly at home, which provides convenience for patients to take drugs quickly and reduces the pressure on medical resources.
Moreover, they are highly stable and do not require special storage and transportation methods. For low- and middle-income countries where medical resources are relatively scarce, they can play an important role in the treatment of patients with COVID-19.
Paxlovid and Molnupiravir, which are currently receiving attention, have shown their efficacy in significantly reducing the risk of hospitalization or death in patients with COVID-19s in clinical trials. They use two different mechanisms to inhibit the replication of the new coronavirus.
Molnupiravir is a nucleoside analogue. The new coronavirus is an RNA virus. A key step in its propagation is to use RNA polymerase to replicate the RNA encoding the viral protein. Molnupiravir is added to the RNA chain as a raw material for synthetic RNA, which will cause the virus to die due to too many errors in the RNA.
Paxlovid works by inhibiting the 3CL protease of the new coronavirus. After the new coronavirus enters the cell, it uses the cell’s protein synthesis mechanism to generate a long polypeptide chain containing a variety of functional proteins.
This long polypeptide chain needs to be cleaved by 3CL protease to produce functional proteins. Paxlovid inhibits the activity of the 3CL protease, making the subsequent RNA replication process of the virus impossible.
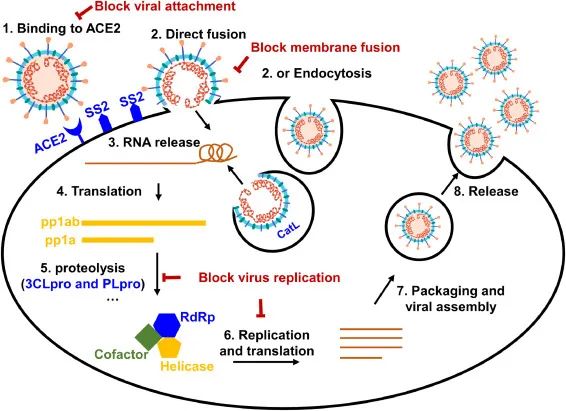
▲Different stages of the new coronavirus replication cycle, Paxlovid targeted protease cleavage step (step 5 in the figure), Molnupiravir targeted RNA replication and translation (step 6 in the figure) (picture source: reference [6])
The rapid development of these two oral antiviral drugs is expected to provide new tools for the treatment of patients with COVID-19. At the same time, scientists are still seeking answers to some scientific questions related to them.
For example, will the widespread use of oral anti-coronavirus drugs lead to the rapid emergence of drug resistance and make them lose their effect soon? The emergence of drug resistance is no stranger to us.
Whether it is antibiotics or antiviral drugs, historical experience has shown that their use will cause bacteria and viruses to develop drug resistance, which is the result of natural selection.
Antiviral therapies to treat HIV virus and hepatitis B virus quickly made the virus strains in the patient’s body resistant.
Unlike antiviral therapies for the treatment of HIV and hepatitis B viruses, oral anti-coronavirus drugs do not need to be taken for a long time.
Their treatment course is usually only 5 days, so it is unlikely to bring long-term selection pressure to the virus and promote drug-resistant virus strains.
The frequency of mutations of the new coronavirus is relatively low, so it may delay the production of resistant mutants.
However, scientists also pointed out that the emergence of resistant virus strains requires long-term and close monitoring.
One of the main ways to solve drug resistance is to use “cocktail” combination therapy.
Because Molnupiravir and Paxlovid use different mechanisms to inhibit the replication of the new coronavirus, in theory, their combined use may bring a stronger effect and prevent the development of drug resistance.
The combination of nucleoside analogs and protease inhibitor antiviral therapy also has early precedents. One of the “cocktail” therapies for the treatment of HIV is the combination of nucleoside analogs and protease inhibitors.
Whether the combination of Molnupiravir and Paxlovid can bring better results still needs to be verified by clinical trials.
In addition to considering their efficacy, whether the combination therapy will cause stronger side effects, thereby limiting the use of the population, is also a question that scientists need to consider.
When it comes to side effects, if you want to use these oral therapies in a wide range of people, their safety needs to be strictly verified.
In a paper previously published in The Journal of Infectious Disease, scientists discovered in vitro cell experiments that Molnupiravir can not only introduce mutations into the genome of the new coronavirus, but also introduce mutations into the genome of mammalian cells.
Whether Molnupiravir will induce genetic mutations in human cells, and how large this potential risk is, is therefore a question of concern to scientists.
In the animal experiments and clinical trials conducted by Merck, the researchers did not find the risk of gene mutations under the normal dosage of the drug.
Scientists also recommend a long-term evaluation of its safety.
At present, the efficacy of these two oral antiviral therapies has only been evaluated in people who have not received the COVID-19 vaccine, so can they be used to treat patients who have a breakthrough infection after receiving the COVID-19 vaccine?
Theoretically, oral antiviral therapy can reduce the level of the new coronavirus in the body and have an effect on preventing the aggravation of the disease.
However, in clinical trials, the effect of treating patients who have been vaccinated with the COVID-19 vaccine may be more difficult to measure.
Because, the current clinical trials test the effect of drugs in preventing severe illness. Among patients with breakthrough infection who have received the COVID-19 vaccine, due to the existing immunity in the body, the proportion of severe illness requiring hospitalization or death is lower.
This also means that it becomes more difficult to observe the efficacy of antiviral therapy.
And theoretically, a result of the COVID-19 vaccine is that the memory B cells and T cells in the human body “remember” the “look” of the virus, and quickly produce neutralizing antibodies when they invade again.
If oral antiviral therapy is not administered in time, the body’s immune response may have controlled the replication of the virus.
However, oral antiviral therapy provides more means and strategies to control the epidemic.
Dr. Jerome Kim, director of the International Vaccine Institute, pointed out that if a new virus strain that can evade the immunity provided by existing vaccines appears somewhere in the world, oral antiviral drugs should be taken before an effective vaccine is available. Therapies can be a powerful tool to inhibit the spread of virus strains.
However, one of the keys to the efficacy of oral antiviral therapy is to use it in the early stage of the patient’s viral infection.
The clinical trials of Molnupiravir and Paxlovid start medication within 5 days or 3 days of the onset of symptoms, respectively.
This poses a challenge to the widespread use of oral anti-coronavirus therapies. It is not enough to have effective treatments.
We also need convenient and fast diagnostic methods to diagnose patients in the early stages of new coronavirus infection and allow them to quickly use effective drugs.
The early end of the COVID-19 epidemic is a process that requires multi-faceted collaboration.
The COVID-19 vaccine, neutralizing antibodies, oral antiviral drugs, nucleic acid testing, and public health measures will all contribute their own strengths.
Reference:
[1] 8 lingering questions about the new Covid pills from Merck and Pfizer. Retrieved November 16, 2021, from https://www.statnews.com/2021/11/15/8-lingering-questions-about-the-new-covid-pills-from-merck-and-pfizer/
[2] PFIZER SEEKS EMERGENCY USE AUTHORIZATION FOR NOVEL COVID-19 ORAL ANTIVIRAL CANDIDATE. Retrieved November 16, 2021, from https://www.pfizer.com/news/press-release/press-release-detail/pfizer-seeks-emergency-use-authorization-novel-covid-19
[3] PFIZER AND THE MEDICINES PATENT POOL (MPP) SIGN LICENSING AGREEMENT FOR COVID-19 ORAL ANTIVIRAL TREATMENT CANDIDATE TO EXPAND ACCESS IN LOW- AND MIDDLE-INCOME COUNTRIES. Retrieved November 16, 2021, from https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-medicines-patent-pool-mpp-sign-licensing
[4] COVID antiviral pills: what scientists still want to know. Retrieved November 16, 2021, from https://www.nature.com/articles/d41586-021-03074-5
[5] Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV. Retrieved November 16, 2021, from https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-arv/what-start-initial-combination-regimens-antiretroviral-naive
[5] Merck’s Antiviral Could Be Just What Covid Was Waiting For. Retrieved November 16, 2021, from https://www.wired.com/story/merck-covid-antiviral-drug-Molnupiravir/
[6] Su et al., (2021). Drug discovery and development targeting the life cycle of SARS-CoV-2. Fundamental Research, https://doi.org/10.1016/j.fmre.2021.01.013
Why many questions about COVID-19 oral drugs still need to be answered?
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



