The mystery of an adenovirus-based COVID-19 vaccine-related ultra-rare blood clot
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
The mystery of an adenovirus-based COVID-19 vaccine-related ultra-rare blood clot
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
The mystery of an adenovirus-based COVID-19 vaccine-related ultra-rare blood clot
An international team of scientists believes that they may have discovered the molecular mechanism behind the extremely rare blood clot associated with the COVID-19 adenovirus vaccine.
Scientists led by Arizona State University, Cardiff University and other teams collaborated with AstraZeneca to study vaccine-induced immune thrombotic thrombocytopenia (VITT), also known as thrombotic thrombocytopenia syndrome (TTS), only Very few people experience this life-threatening condition after being vaccinated with Oxford-AstraZeneca or Johnson & Johnson vaccines.
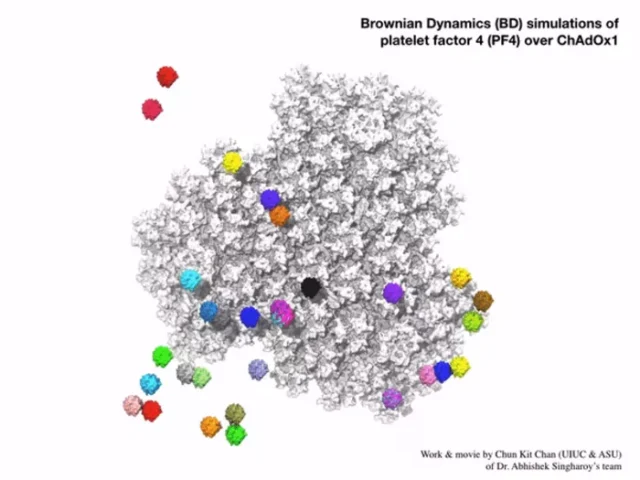
This computer simulation showed a group of platelet factor 4 protein interacting with the electrostatic surface of Oxford vaccine.
Image: Arizona State University Chun Kit Chan
An international team of scientists believes that they may have discovered the molecular mechanism behind the extremely rare blood clot associated with the COVID-19 adenovirus vaccine.
Scientists led by Arizona State University, Cardiff University and other teams collaborated with AstraZeneca to study vaccine-induced immune thrombotic thrombocytopenia (VITT), also known as thrombotic thrombocytopenia syndrome (TTS), only Very few people experience this life-threatening condition after being vaccinated with Oxford-AstraZeneca or Johnson & Johnson vaccines.
“The mechanism that causes this, called vaccine-induced immune thrombotic thrombocytopenia (VITT), is not yet clear,” said Abhishek Singharoy, a scientist at Arizona State University and a liaison author of the study, and he co-led An international effort was made to sort out the details.
Therefore, a team quickly organized to try to understand the problem more clearly.
They worked together to solve the structural biology of vaccines and used Arizona State University’s new cryo-electron microscope equipment and the state-of-the-art Titan Krios machine at Arizona State University’s Eyring Materials Center to observe the molecular details that might work.
A very detailed analysis of the AstraZeneca vaccine was conducted to understand whether this extremely rare side effect is related to the viral vectors used in many vaccines, including the Oxford/AstraZeneca and Johnson & Johnson vaccines.
Their findings indicate that the viral vector-in this case, an adenovirus used to transport the genetic material of the coronavirus into the cell-and the way it binds to platelet factor 4 (PF4) may be The underlying mechanism.
Scientists believe that in very rare cases, the viral vector may enter the blood and bind to PF4, and then the immune system will treat this complex as a foreign object.
They believe that this misaligned immunity may lead to the release of antibodies against PF4. PF4 binds to platelets and activates the platelets, causing the platelets to clump together and triggering a small number of human blood clots after the vaccine.
The author said: “It is really important to comprehensively study the vector-host interaction of vaccines at the mechanical level.
This will help understand how vaccines produce immunity and how it may cause any rare adverse events such as VITT.”
Their findings are published today in the international journal Science Advances.
Professor Allen Parker, an adenovirus medical application expert at Cardiff University School of Medicine, said: “VITT will only happen in extremely rare cases, because it takes a series of complex events to trigger this extremely rare side effect. Our data It is confirmed that PF4 can bind to adenovirus, which is an important step in revealing the mechanism of VITT. Establishing a mechanism can help prevent and treat this disease.”
“We hope that our findings can be used to better understand the rare side effects of these new vaccines, and it is possible to design new and improved vaccines to reverse the trend of this global pandemic.”
Vaccines from AstraZeneca and Johnson & Johnson both use adenovirus to carry the spike protein from the coronavirus into the human body, thereby triggering a protective immune response.
When both vaccines showed the extremely rare side effects of VITT, scientists wanted to know whether the viral vector played a role.
Another important clue is that neither Moderna nor Pfizer’s vaccines have shown this effect. Both vaccines are made by a completely different technology called mRNA vaccines.
The team used cryo-electron microscopy technology to quickly freeze ChAdOx1, the adenovirus used in AstraZeneca’s vaccine, and bombard it with electrons to produce microscopic images of the vaccine components.
Then, they were able to observe at the atomic level the structure of the outer protein cage of the virus (that is, the virus capsid), as well as other key proteins that allow the virus to enter the cell.
In particular, the team outlined the structure and receptor details of ChAdOx1, which is adapted from chimpanzee adenovirus Y25, and how it interacts with PF4.
They believe that it is this particular interaction-and how it is presented to the immune system-that may prompt the body’s own defense system to treat it as foreign and release antibodies against this own protein.
The research team also used Singh Roy’s computational model to show that one of the ways the two molecules are tightly bound is through electrostatic interaction.
The team showed that ChAdOx1 is mainly electronegative. This makes the protein work like the negative electrode of a battery and can attract other positively charged molecules to its surface.
The first author of the study, Dr. Alexander Baker, said: “We found that ChAdOx1 has a strong negative charge.
This means that viral vectors can attract oppositely positively charged proteins, such as PF4, like a magnet.”
“Then we found that the size and shape of PF4 are just right.
When it is close to ChAdOx1, it can bind between the negatively charged parts of the ChAdOx1 surface, which is called a hexagon.”
The research team hopes that if they can better understand the causes of rare VITT, they can further understand the possible changes in the development of next-generation vaccines and therapies for vaccines and other therapies that rely on the same technology.
“With a better understanding of the mechanism of interaction between PF4 and adenovirus, there is an opportunity to design the outer shell of the vaccine, the capsid, to prevent this interaction with PF4.
Modifying ChAdOx1 to reduce the negative charge may reduce thrombocytopenia Chance of syndrome thrombosis,” Baker said.
The team likened it to the “two birds with one stone” effect. The key contact points of individual amino acids necessary for the interaction of capsid protein with PF4 can be removed or replaced.
“Modifying the ChAdOx1 hexon to reduce its electronegativity may solve two problems at the same time: reduce the tendency to cause VITT to a lower level, and reduce the existing level of immunity, thereby helping to maximize the induction of strong immunity Opportunity to react.”
The British Medicines and Healthcare Products Administration (MHRA) and the US Centers for Disease Control and Prevention (CDC) continue to recommend that vaccination is the best way to protect people from COVID-19, and the benefits far outweigh the risks of any known side effects.
Reference:
ChAdOx1 interacts with CAR and PF4 with implications for thrombosis with thrombocytopenia syndrom
The mystery of an adenovirus-based COVID-19 vaccine-related ultra-rare blood clot
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.