How to deal with low expression of HER2 in breast cancer?
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
How to deal with low expression of HER2 in breast cancer?
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
How to deal with low expression of HER2 in breast cancer? New antibody-conjugated drugs perform well
Breast cancer is the most common type of cancer in women, and 80% of patients are HER2-negative. This type of HER2-expressing breast cancer has no approved precision therapy.
Breast cancer is the most common type of cancer in women and the leading cause of cancer-related death in women. According to statistics, about 20% of breast cancers are HER2 positive. With the continuous approval of HER2-targeted drugs, the survival rate of patients with HER2-positive metastatic breast cancer has been significantly improved.
However, 80% of breast cancer patients are HER2-negative, and more than 40% of these patients are cancer cells that express HER2 at low levels in the form of surface antigens.
There is currently no approved precision therapy for this type of HER2-low expressing breast cancer.
Existing clinical guidelines classify these patients as HER2-negative, and chemotherapy remains the only treatment option. The patient’s disease progresses after chemotherapy.
Recently, the antibody-drug conjugate (ADC) Enhertu achieved positive results in a pivotal Phase 3 trial in metastatic breast cancer with low HER2 expression.
The data showed that Enhertu significantly improved progression-free survival (PFS) and overall survival (OS) compared with chemotherapy, regardless of the patient’s hormone receptor (HR) status.
This is the world’s first Phase 3 clinical study of HER2-targeted therapy in patients with metastatic breast cancer with low HER2 expression. It promises to rewrite breast cancer disease classification and treatment standards.
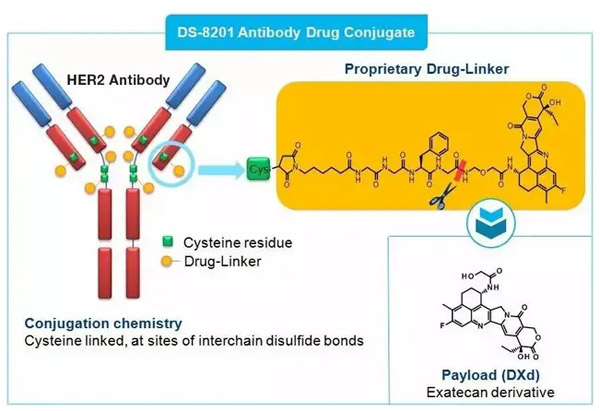
The active ingredient of Enhertu is trastuzumab deruxtecan, a HER2-targeting antibody conjugated drug. It links the human HER2 antibody trastuzumab to a novel topoisomerase 1 inhibitor via a tetrapeptide linker to target cancer cells and deliver the drug inside the cells. Reduced systemic exposure to cytotoxic agents compared to usual chemotherapy.
Enhertu was previously approved by the FDA for the third-line treatment of previously treated patients with unresectable or metastatic HER2-positive breast cancer.
This study is a global, randomized, open-label, registration phase 3 trial involving 480 HR-positive and 60 HR-negative patients with unresectable and/or metastatic breast cancer with low HER2 expression.
To assess the efficacy and safety of Enhertu versus chemotherapy agents (eg, capecitabine, eribulin, gemcitabine, paclitaxel, or nab-paclitaxel), patients were randomized 2:1 to either Enhertu or chemotherapy Group.
The primary endpoint was PFS in HR-positive patients, and key secondary endpoints included: PFS in all patients, OS in HR-positive patients, and OS in all patients. Other secondary endpoints included duration of response and safety.
The results of the interim analysis showed:
1. In previously treated patients with HR-positive HER2-low metastatic breast cancer, treatment with Enhertu demonstrated higher progression-free survival (PFS) compared to standard chemotherapy.
2. The trial also met the key secondary endpoint of PFS: significant prolongation of PFS regardless of HR status.
3. The key secondary endpoint of overall survival (OS) was also met: OS was significantly prolonged in patients with HR-positive disease and in patients regardless of HR status.
4. The safety of Enhertu is consistent with previous clinical trials, and no new safety issues were found.

Previously, HER2-directed therapy has never shown benefit in patients with low HER2 metastatic breast cancer, and Enhertu’s findings are a huge advance that could reshape how breast cancer is classified and treated.
Expert introduction:
Dr. Linnea Chap, a gynecological oncologist at Good Medical Friend Medical Network, introduced that low HER2 expression can occur in patients with HR-positive or HR-negative breast cancer, and the current medical consensus believes that patients with HER2 expression at this level are not eligible for HER2-targeted therapy. standard. The breakthrough results achieved by Enhertu this time are expected to break the medical community’s understanding of the population receiving HER2-targeted therapy.
Today, breast cancer has truly bought into the era of “precision therapy”. It is hoped that this ADC drug will make more progress in clinical research and bring survival benefits to patients with this type of breast cancer as soon as possible.
Reference:
https://www.onclive.com/view/trastuzumab-deruxtecan-significantly-improves-pfs-os-in-her2-low-metastatic-breast-cancer
How to deal with low expression of HER2 in breast cancer?
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



