Sanofi GSK COVID-19 vaccines may join the booster shot soon.
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Sanofi GSK COVID-19 vaccines may join the booster shot soon.
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
The counterattack of traditional vaccine giants: Sanofi GSK COVID-19 vaccines may join the booster shot soon.
Before the outbreak of the COVID-19 epidemic, Sanofi and GSK were both among the top four vaccine companies in the world.
Sanofi is a large flu vaccine manufacturer. Moderna’s trial of simultaneous vaccination of the COVID-19 vaccine and flu vaccine is to play with Sanofi’s flu vaccine. And GSK is best known for its shingles vaccine.
But the two have made little achievements in the development of the COVID-19 vaccine.
However, unlike Merck, another four major vaccine manufacturers (one of the top four is Pfizer), Sanofi and GSK, especially Sanofi, are eager to do something.
The two companies began to cooperate on a recombinant protein vaccine very early , Sanofi was responsible for the recombinant protein, and GSK provided the adjuvant. Because the technology of the two companies is very mature, they were very optimistic at the beginning, and the governments of Europe and the United States also invested money and signed contracts of intent. Later, it was very unsatisfactory.
A clinical trial found that the induced immune response was not good, and the antibody titer was low. Had to rework and adjust the amount of antigen, resulting in a significant slowdown in progress.
Due to the slow progress, several mRNA and adenovirus vaccines were already on the market by the time the vaccines of these two companies started Phase III clinical trials.
Therefore, although Sanofi GSK’s vaccine is still undergoing Phase III trials for the initial vaccination population, the main direction is not to give the first shot to people who have not been vaccinated, but to consider booster shots.
In late February, Sanofi and GSK announced the protective effect data of the Phase III clinical trial, and provided the data of the booster shot, and the results looked good. On February 23, the two announcements decided to submit a listing application.
The third phase of the clinical trial was done in 10,000 people, with two injections , three weeks apart .
Symptomatic protection is 57.9% , which doesn’t seem like a good number, but there’s a Delta during the trial and an Omicron later, so it’s not bad.
According to the press release, the early stage is 77% effective during the delta period . Comparable to past vaccines such as mRNA vaccines.
75 % of moderate to severe cases are prevented, and 100% of hospitalization and death are prevented . However, there are very few severe deaths, and the confidence interval is relatively large.
A total of 14 cases were moderate to severe, 3 in the vaccine group and 11 in the placebo group.
Now doing clinical trials, the popular virus strains are different from those encountered by several mRNA vaccines and adenovirus vaccines in the early days, so the effectiveness figures cannot be directly compared – there are also problems in comparing different clinical trials .
But we can synthesize more aspects of the data, such as immunological data. To analyze and compare the new vaccine and the old vaccine.
From the perspective of antibody formation, this recombinant protein vaccine is really good. Compared with mRNA vaccine recipients, the titer of two doses of mRNA vaccine was 1653 and the titer of two doses of this vaccine was 3711 when neutralizing antibodies (against the original strain) were made in the same laboratory.
Neutralizing antibody titers in different laboratories and using different methods will produce very different numbers, but this is done in the same laboratory, so it can be compared.
Of course, there are still potential biases such as the dissimilarity of the two types of vaccine recipients tested, but even taking these into account, it can still be said that the immune response of the Sanofi GSK vaccine is not bad .
Another important trial is the booster shot. Judging from the press release, this trial used a bivalent vaccine, in addition to the original virus strain, the antigen of the Beta strain was also used.
Regardless of whether the mRNA vaccine or the adenovirus vaccine was previously vaccinated, another shot of the Sanofi GSK vaccine can improve the antibody a lot .
However , the biggest improvement . The mRNA vaccine, whether Pfizer or Moderna, has been raised to 7000. Sanofi GSK homologous vaccination starts at more than 300 (similar to the starting point of the mRNA vaccine in the trial), and the third shot reaches 28,000:
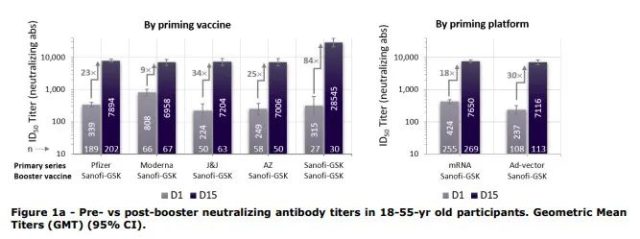
The three-shot antibody titer of the elderly over 56 years old also exceeded 20,000. However, the number of Sanofi GSK third-dose groups is relatively small, with less than 30 people in both age groups.
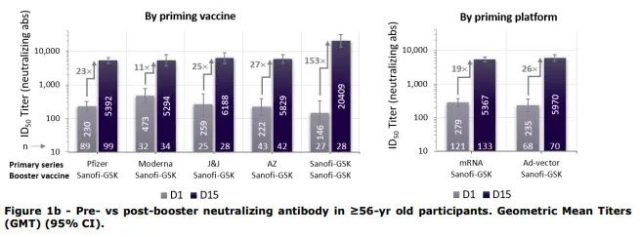
If such high antibody titers are common, they are well worth further study .
In the current vaccination, the three-shot mRNA vaccine seems to be the peak of humoral immunity (antibody), and the ability to protect Omicron from infection is still insufficient.
If the third-dose of Sanofi GSK can reach a higher peak, does it mean that Omicron can also have a better anti-infection effect?
In the past research on the combination of enhanced injections, there is not much difference between the mixed injection of the third mRNA vaccine and the three-injection homologous mRNA vaccine.
The homologous third-dose of Sanofi GSK is much higher than the mixed fight. What is the reason? From the perspective of immunology and vaccine research and development, it is an interesting and important question.
From the perspective of the characteristics of the COVID-19 disease itself, we must focus on improving the immune protection of high-risk groups , such as the elderly, immunosuppressed people, and people with various basic diseases.
If there was a vaccine with better immunogenicity and higher antibody formation, it would make a lot of sense for these populations.
In general, the preliminary data of Sanofi and GSK are good, but the published data is only partial, and the number of people involved is smaller than that of mRNA vaccine trials published in the past. We will have to wait for the detailed data to make a better judgment.
Some people believe that the safety of recombinant protein vaccines is guaranteed on the grounds that recombinant protein vaccines are traditional technologies.
This statement is problematic. The safety of each vaccine needs to be independently evaluated, and it cannot be assumed that there is no problem because of the traditional technology platform.
In particular, the actual differences between different recombinant protein vaccines may be very large . For example, in terms of the proteins used, some COVID-19 manufacturers use multimer design, and some use S2P mutation.
In addition to the protein, an important component of this type of vaccine is the adjuvant. Different adjuvants will make the immune response very different, and the safety should be evaluated separately and cannot be confused.
GSK offers the A03 adjuvant (also used in a recombinant protein vaccine from Medicago, which has just been approved in Canada), which is not the same as the adjuvant used by another company, Novavax.
A small episode, A03 adjuvant is GSK’s “pandemic adjuvant” used to make vaccines for pandemic influenza such as the 2009 H1N1 flu vaccine.
In the use of such influenza vaccines, some European countries have found an increased risk of a neurological disorder called narcolepsy in those vaccinated with A03-adjuvanted vaccines compared with unadjuvanted vaccines.
It is unclear whether this increased risk is causally related to vaccination, nor is it related to A03 or to other components of the vaccine, such as the H1N1 antigen.
But this at least tells us that the safety analysis of each vaccine, no matter what kind of technology, must be considered independently, and it is not possible to simply copy the homework or even do not do the homework .
Going back to the vaccines of Sanofi and GSK, considering that the COVID-19 vaccine may need to be regularly boosted in the future – not necessarily everyone needs it, but some high-risk groups may be so, then the safety data must start to consider how common adverse reactions are , the side effects can be smaller.
Both announced neutralizing antibodies against the original virus strain, and the mutant strain antibodies are related to the original strain antibodies, but the Omicron antibody is still worthy of attention.
Although Sanofi GSK has released less information, it is always good to have one more choice.
The booster shot of the bivalent vaccine also verifies the feasibility of this platform for multivalent vaccines.
Of course, when and how this vaccine can be used depends on the analysis and consideration of regulatory agencies such as the FDA.
Sanofi GSK COVID-19 vaccines may join the booster shot soon.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



