Sanofi and GlaxoSmithKline Announced Clinical Trial Data of COVID-19 Vaccines for Basic Immunization and Booster shot!
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Sanofi and GlaxoSmithKline Announced Clinical Trial Data of COVID-19 Vaccines for Basic Immunization and Booster shot!
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Sanofi and GlaxoSmithKline Announced Clinical Trial Data of COVID-19 Vaccines for Basic Immunization and Booster shot!
GlaxoSmithKline (GSK) and Sanofi (Sanofi) recently jointly announced that they will submit final data from the global VAT02 booster trial of the Sanofi-GSK vaccine, as well as the VAT08 Phase 3 primary series efficacy trial. data as the basis for the regulatory authorization for this COVID-19 vaccine.
The public health benefits of a Sanofi-GSK vaccine based on a refrigerator temperature-stabilized adjuvant protein are strongly supported by robust immune response induction and favorable safety profile in multiple settings.
Data from the final analysis of the global VAT02 booster trial showed that the Sanofi-GSK booster vaccine was effective across a variety of vaccine platforms and age groups in subjects who had been immunized with a series of authorized mRNA vaccines or adenovirus vaccine basal immunizations Neutralizing antibody levels induced in 18-30-fold were significantly increased . When the Sanofi-GSK vaccine was used for 2 basal immunizations followed by a booster dose, neutralizing antibody levels were induced to increase 84- to 153-fold compared to pre-boost levels (see Figures 1a and 1b below).
The Sanofi-GSK vaccine produces high levels of neutralizing antibodies with a GMT of 3711 units when used in 2-dose basal immunizations. For comparison, a pool of sera from volunteers of the same age group who had received 2 doses of an approved high-potency mRNA vaccine, measured simultaneously in the same laboratory, showed a GMT of 1653 units.
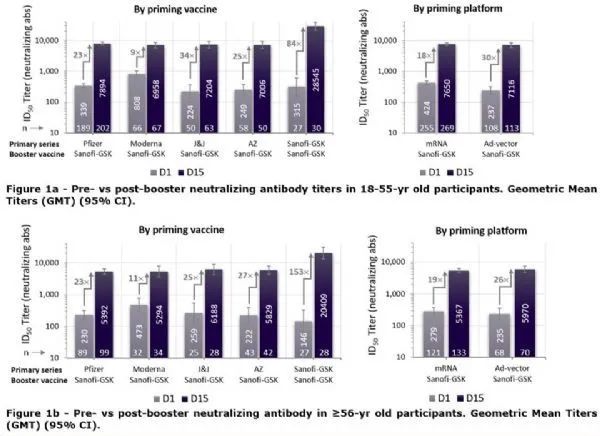 VAT02 booster shot test data
VAT02 booster shot test data
Data from the VAT08 Phase 3 Basic Immunization Efficacy Trial showed that 2 doses of Sanofi-GSK vaccine were 57.9% (95% CI: 26.5, 76.7) effective in preventing any symptomatic COVID-19 disease in a seronegative population .
In the seronegative population, the Sanofi-GSK vaccine was 100% effective in preventing severe illness and hospitalization (after 1st dose: 0 vs 10; after 2nd dose: 0 vs 4), preventing moderate The response rate to severe disease was 75% (3 vs 11).
While sequencing is still in progress, early data suggest that, consistent with the expected vaccine efficacy, it is 77% effective in preventing symptomatic COVID-19 disease associated with any Delta variant .
In both studies, the Sanofi-GSK vaccine, used as a 2-dose base immunization and as a 1-dose booster, was well tolerated in the young and elderly populations with no safety concerns.
GlaxoSmithKline and Sanofi are in discussions with regulators, including the US FDA and EU EMA, and plan to submit full data generated from the Sanofi-GSK vaccine to support regulatory authorization.
Reference:
Sanofi and GSK to seek regulatory authorization for COVID-19 vaccine
Sanofi and GlaxoSmithKline Announced Clinical Trial Data of COVID-19 Vaccines for Basic Immunization and Booster shot!
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



