Moderna: HIV Trimeric mRNA Vaccine Phase I Clinical Completed First Dosing
- Why Botulinum Toxin Reigns as One of the Deadliest Poisons?
- FDA Approves Pfizer’s One-Time Gene Therapy for Hemophilia B: $3.5 Million per Dose
- Aspirin: Study Finds Greater Benefits for These Colorectal Cancer Patients
- Cancer Can Occur Without Genetic Mutations?
- Statins Lower Blood Lipids: How Long is a Course?
- Warning: Smartwatch Blood Sugar Measurement Deemed Dangerous
Moderna: HIV Trimeric mRNA Vaccine Phase I Clinical Completed First Dosing
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Moderna: HIV Trimeric mRNA Vaccine Phase I Clinical Completed First Dosing.
On March 14, 2022 Moderna, Inc. announced the first recipient of its human immunodeficiency virus (HIV) trimeric mRNA vaccine (development code: mRNA-1574) in a Phase I clinical trial.
The subject has completed the drug administration.
“Developing a vaccine that induces sustained levels of protection against HIV neutralizing antibodies in humans has historically been elusive,” said Moderna President Stephen Hoge, MD . “At Moderna, we believe mRNA provides a new way to address this challenge.
With the launch of our second HIV vaccine trial, we are advancing our strategy to leverage multiple mRNA-encoded native HIV-like trimers and our mRNA platform to accelerate the development of protective HIV vaccines.”
Phase I trial HVTN 302
This open-label, multicenter, randomized phase I trial (HVTN 302) was designed to evaluate the safety and immunogenicity of the HIV trimeric mRNA vaccine mRNA-1574 . The trial is expected to enroll approximately 100 HIV-negative adults between the ages of 18 and 55.
The trial was funded by the Division of AIDS ( D AIDS ) of the National Institute of Allergy and Infectious Diseases ( NIAID) of the National Institutes of Health ( NIH) .
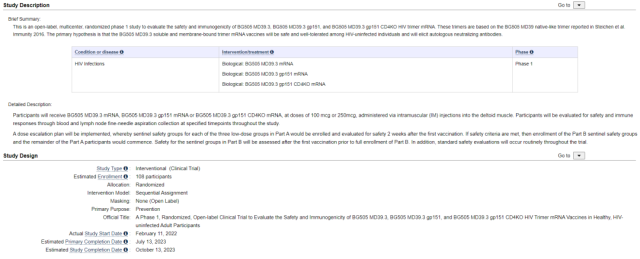
(NCT05217641 Screenshot from: clinicaltrials.gov)
“It’s great to see that mRNA, a key platform for COVID vaccines, is making its way into the HIV vaccine space,” said Larry Corey, PhD, Principal Investigator, HIV Vaccine Trials Network (HVTN) Operations Center . The field uses mRNA to pave the way. ”
Moderna in HIV
HIV is the virus that causes Acquired Immune Deficiency Syndrome (commonly known as “AIDS”, AIDS), a lifelong progressive disease with no effective cure. Currently, approximately 38 million people worldwide are infected with HIV.
Moderna is currently advancing two HIV preventive vaccine strategies based on germline targeting* and immune-focused approaches, in addition to mRNA-1574 described above , Moderna is also working on IAVI and Bill & Melinda Gates With the support of the foundation, the phase I clinical trials of HIV vaccine mRNA-1644 and mRNA-1644v2-core were carried out.
*In 2019, SCIENCE published a paper titled A generalized HIV vaccine design strategy for priming of broadly neutralizing antibody responses, introducing an HIV vaccine that initiates induction of broadly neutralizing antibodies (bnAbs) through germ cell-targeted antigens Strategy.
Reference:
[1] www.modernatx.com
[2] Jon M. Steichen et al. A generalized HIV vaccine design strategy for priming of broadly neutralizing antibody responses. Science, 2019, doi:10.1126/science.aax4380.
Moderna: HIV Trimeric mRNA Vaccine Phase I Clinical Completed First Dosing.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



