WHO announced the composition of influenza vaccine strains in 2022-2023
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
WHO announced the composition of influenza vaccine strains in 2022-2023
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
WHO announced the composition of influenza vaccine strains in 2022-2023. What kind of research should we conduct?
From the Spanish flu in 1918 to the present, the flu has been one of the strongest opponents of mankind for more than 100 years. As an RNA virus, the influenza virus, despite its simple structure, appears to be more mutated and transmissible than humans can respond.
The World Health Organization (WHO) announces the components of influenza vaccines for the northern and southern hemispheres of the next season in the first and second half of each year [1] .
The downfall of the official organization also represents the importance of influenza vaccine research.
2022-2023 Northern Hemisphere seasonal influenza vaccine recommended components:
Quadrivalent chicken embryo cultured vaccine
- an A/Victoria/2570/2019 (H1N1)pdm09-like virus;
- an A/Darwin/9/2021 (H3N2)-like virus;
- a B/Austria/1359417/2021 (B/Victoria lineage)-like virus;
- a B/Phuket/3073/2013 (B/Yamagata lineage)-like virus.
Tetravalent cell culture or recombinant protein vaccine
- an A/Wisconsin/588/2019 (H1N1)pdm09-like virus;
- an A/Darwin/6/2021 (H3N2)-like virus;
- a B/Austria/1359417/2021 (B/Victoria lineage)-like virus;
- a B/Phuket/3073/2013 (B/Yamagata lineage)-like virus.
Trivalent chicken embryo cultured vaccine
- an A/Victoria/2570/2019 (H1N1)pdm09-like virus;
- an A/Darwin/9/2021 (H3N2)-like virus;
- a B/Austria/1359417/2021 (B/Victoria lineage)-like virus.
Trivalent cell culture or recombinant protein vaccine
- an A/Wisconsin/588/2019 (H1N1)pdm09-like virus;
- an A/Darwin/6/2021 (H3N2)-like virus;
- a B/Austria/1359417/2021 (B/Victoria lineage)-like virus.
Influenza virus recombinant protein is a key material for vaccine production
Recombinant influenza protein can be used in all aspects of vaccine development, such as vaccine content detection, vaccine biological titer detection, and toxicological experimental research. Among them, the key target proteins play an important role in research, such as HA, NA, NP and other important target proteins (as shown in Figure 1).
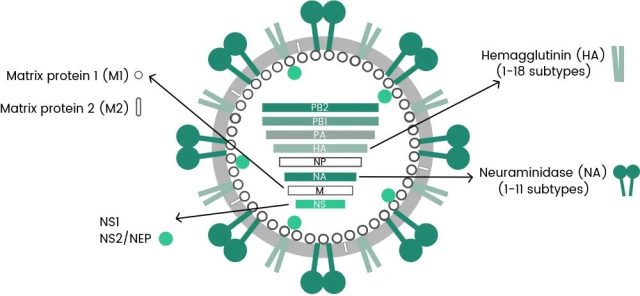
Figure 1: HA, NA, NP-related protein functions (from wiki)
Using recombinant protein for ELISA detection can analyze the level of total antibody and neutralizing antibody in serum after vaccine immunization, and use recombinant protein as a control substance to detect vaccine content.
1. New DNA vaccines:
New DNA vaccines have certain advantages in dealing with influenza virus mutation. Andersen et al. [2] directly targeted influenza antigens to antigen-presenting cells (APCs) and showed that the efficacy of DNA vaccines was comparable to traditional vaccine technologies (Figure 2).
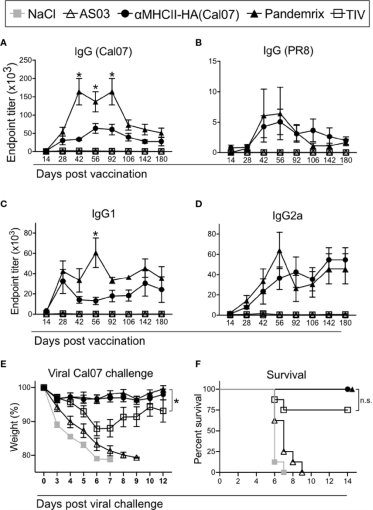 Figure 2: Using recombinant influenza HA protein to detect serum antibody levels in mice after vaccine immunization [2]
Figure 2: Using recombinant influenza HA protein to detect serum antibody levels in mice after vaccine immunization [2]
2. Universal vaccine:
Universal vaccine is an important idea for influenza vaccine development. Bangaru et al [3] found that the antibody FluA-20 can enter the stable trimeric HA region of influenza protein and divide it, so that the virus cannot spread between cells (as shown in Figure 3).
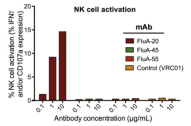
Figure 3: Using recombinant influenza HA protein to confirm that FluA-20 can activate NK cells and induce ADCC activity in vitro [3]
Influenza virus recombinant protein is a key target for drug screening
HA and NA proteins play a key role in drug discovery and development.
HA protein mediates the fusion of influenza virus and host cell membrane, NA protein promotes the release of mature virus particles, and enzymes involved in the responsibility and transcription of viral RNA are all targets for antiviral drug development.
For example, drugs targeting NA include oseltamivir and zanamivir, and drugs targeting HA include arbidol. T
he use of recombinant proteins for pharmacodynamic analysis or screening is a commonly used technology in drug development. The Korea Institute of Chemical Technology [4] used the influenza NA protein of Yiqiao Shenzhou to conduct ceramidase inhibition experiments, and obtained ideal research results.
It can be used as a raw material for serological detection in diagnostic reagents
The current immunological detection methods for influenza virus mainly include serological detection methods and antigen detection methods: antigen detection methods are more suitable for rapid on-site detection and primary screening; serological detection methods use recombinant proteins to detect antibodies in serum. Methods are mostly used in retrospective analysis.
Figure 4. Recombinant NP proteins that can be used to screen antibodies for influenza detection in diagnostic feedstocks.
 Figure 4: Serological assay protocol using recombinant influenza NP protein
Figure 4: Serological assay protocol using recombinant influenza NP protein
Three important target proteins in influenza virus research
From the above cases, we can see that in influenza virus-related vaccines, drugs and even diagnostic reagents, key target proteins have played a significant role. We also summarized the HA, NA, and NP just mentioned for you. Several influenza virus research key protein information to help you better understand and apply. (As shown in Table 1)
Table 1: Key Protein Information Sheet for Influenza Virus Research
References:
[1] https://www.who.int/news/item/25-02-2022-recommendations-announced-for-influenza-vaccine-composition-for-the-2022-2023-northern-hemisphere-influenza-season
[2] Andersen, et al. Pandemic Preparedness Against Influenza: DNA Vaccine for Rapid Relief.[J]. Frontiers in Immunology.2021.747032.
[3] Bangaru S , et al. A Site of Vulnerability on the Influenza Virus Hemagglutinin Head Domain Trimer Interface[J]. Cell, 2019.
[4] Kim M , et al. Inhibition of influenza virus internalization by (-)-epigallocatechin-3-gallate.[J]. Antiviral Research, 2013, 100(2):460-472.
WHO announced the composition of influenza vaccine strains in 2022-2023.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



